Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cultivos Tropicales
versión On-line ISSN 1819-4087
cultrop vol.42 no.4 La Habana oct.-dic. 2021 Epub 30-Dic-2021
Original article
Effect of a bioproduct application in early stages of corn (Zea mays L.) cultivation
1Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
2Facultad de Agronomía, Universidad Agraria de La Habana “Fructuoso Rodríguez Pérez”, (UNAH) carretera a Tapaste y Autopista Nacional, San José de las Lajas, Mayabeque, Cuba, CP 32 700
Two experiments were conducted to evaluate the QuitoMax® application effectiveness in early stages of corn (Zea mays L.) crop development. The first experiment consisted of evaluating QuitoMax® effect on the germination stage, for which germination was evaluated on petri dishes, sowing 10 corn seeds of the Jíbaro cultivar/plate. These were moistened with 1 mL of QuitoMax®/seed with different concentrations (treated groups) or distilled water (control). The concentrations were: 0.05, 0.5 and 1 g L-1 of QuitoMax®. The second experiment consisted of different forms of QuitoMax® application, in early stages of corn crop, for which sowing was carried out in polyethylene bags, seeds were embedded and the best QuitoMax® concentrations found in the previous trial were applied foliarly. Germination, stem and root length, plant height and plant dry mass were evaluated. Result analysis showed a better response of plants when they received the combination of seed imbibition with QuitoMax® foliar spraying at a concentration of 1 g L-1.
Key words: biostimulants; polymers; seeds
INTRODUCTION
Corn (Zea mays L.) is a cereal of great preference and high consumption in the world, both as fresh and processed product 1, due to its nutritional properties, it is a food that contains many carbohydrates and due to its extreme adaptability it has become the most produced food worldwide.
This crop extracts large amounts of nutrients from the soil, so it is necessary to apply adequate fertilization to cover its nutritional requirements 2. Like other cereals, it is characterized as an intensive crop with large and increasing applications of chemicals, which has been reported as a worrying trend in Latin America 3.
Corn is currently grown in all provinces of the country and it is one of the priorities of the state's agrarian policies; the productivity of these cultivars does not exceed 1.44-2.35 t ha-1 on average 4, and one of the limitations of its production is the incidence of pests that frequently reduce yields, despite the fact that plants resist their attacks 5.
The Cuban state is making efforts to increase the production of this crop, since yields continue to be scarce, so it is necessary to increase its production and yield in all agricultural scenarios 6. In spite of the efforts made by agriculture to increase yields in the crop, these present a national average of 2.25 t ha-1, far from the world average, with values around 4.50 t ha-1 (7.
At world level, and especially in Cuba, hard work is being done in the search for biostimulants and organic biofertilizers that allow plants to overcome stress situations in adverse environmental conditions, favoring growth, development and yield, with a decrease in the use of chemical substances 8.
However, it has recently been established that there are natural substances called biostimulants, which when applied to seeds and plants, can act as accelerators of cell metabolism and positively influence growth, development and protection against plant diseases, leading to a significant increase in yield and fruit health 9,10. Among the best known biostimulants is chitosan, derived from chitin, an active ingredient of natural origin, biodegradable and environmentally friendly, which is obtained from the Department of Plant Physiology and Biochemistry of the National Institute of Agricultural Sciences (INCA).
Chitosan is a very abundant natural polymer extracted from the exoskeleton of crustaceans 11. In this regard, formulations such as QuitoMax®, derived from chitosan, have been successfully used to stimulate yield and its components in beans and potatoes 12,13.
Taking into account the QuitoMax® potential on plant growth and development, our aim was to evaluate the QuitoMax® application effectiveness in early stages of corn (Zea mays L.) crop development.
MATERIALS AND METHODS
QuitoMax® effect on the germination phase of corn seed.
The research was carried out at the Department of Plant Physiology and Biochemistry of INCA, in a growth chamber. A completely randomized experimental design was used. The evaluation of in vitro germination was carried out on 16 mm diameter Petri dishes, sowing 10 corn seeds/plate of Jibaro cultivar and 4 plates/treatment (n=40). Treatments were applied at a rate of 1 mL/seed of QuitoMax® at different concentrations (treated groups) or distilled water (control group), as a substrate on previously sterilized paper (Table 1).
Table 1 Description of treatments studied
| Treatments | Description |
|---|---|
| 1 | Control (distilled water) |
| 2 | QuitoMax® 1 g L-1 |
| 3 | QuitoMax® 0,5 g L-1 |
| 4 | QuitoMax® 0,05 g L-1 |
The germination plates were maintained under controlled conditions of temperature (20.1-22.0 °C) and relative humidity (50-64 %), conditions that were monitored daily using a thermohygrometer (Fisher Scientific). Daily observation was also carried out to determine the emergence time of radicles during a period of 15 days. The experiment was replicated twice over time.
The rest of measurements were taken 15 days after the test starting. After this time, germinated seeds were counted to determine the relative germination percentage, root length (cm), stem length (cm) and plant height (cm).
Effect of different forms of Quitomax® application in early stages of the corn crop
Corn seeds of Jíbaro variety were used, whose seeds were supplied by INCA's Department of Genetics and Plant Breeding, and the experiment was carried out under semi-controlled conditions, with best results in the studies conducted on seed germination (Table 2).
Table 2 Description of the treatments studied
| Treatments | Description |
|---|---|
| 1 | Control (imbibition with distilled water) |
| 2 | Seed imbibition 1 hour (1 g L-1) |
| 3 | Imbibition of the seed 1 hour (0.5 g L-1) |
| 4 | Foliar spraying (1 g L-1) (dosage 10 ml x plant) |
| 5 | Foliar spraying (0.5 g L-1) (dose 10 ml x plant) |
| 6 | Imbibition of the seed and foliar spraying (1 g L-1) |
| 7 | Imbibition of the seed and foliar spray (0.5 g L-1) |
To carry out the experiments, polyethylene bags of 7 kg capacity were used with a soil classified as agrogenic leached red Ferrallitic 14, collected in areas of the National Institute of Agricultural Sciences (INCA), ten bags were used for each treatment, five seeds were sown per bag and seven days after the plants emerged, only one was left in each bag, with a completely randomized design and irrigation was performed every two days. This experiment was repeated three times over time (n=30).
Foliar sprays were made 15 and 30 days after plant emergence. At 15 days after the first foliar spray, root length (cm) and plant height (cm) were measured on five plants per treatment; at 45 days after emergence, root length, plant height (cm) and dry mass (g) were determined.
RESULTS Y DISCUSIÓN
Efecto del QuitoMax® en la fase de germinación de la semilla de maíz
Seed germination is an oxidative process, influenced by multiple factors. Figure 1 shows the result of the germination percentage in corn seedlings of Jibaro cultivar, under controlled conditions. It can be observed that in the first five days after germination (DAG), QuitoMax® concentrations used (T2, T3 and T4) failed to stimulate germination above the control (T1), as there were no significant differences between treatments, with the lowest value obtained in the concentration of 0.05 g L-1 (v:v) corresponding to treatment T4.
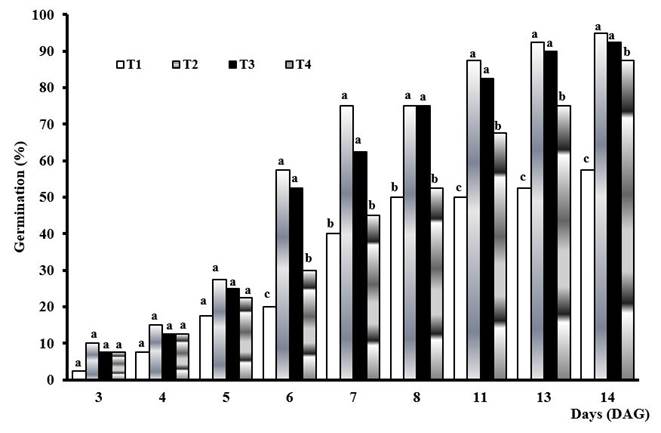 Means of treatments with equal letters do not differ significantly with p<0.05 according to Duncan.T1-Control,T2- Q:1 g L-1, T3- Q:0,5 g L-1, T4- Q:0,05 g L-1
Means of treatments with equal letters do not differ significantly with p<0.05 according to Duncan.T1-Control,T2- Q:1 g L-1, T3- Q:0,5 g L-1, T4- Q:0,05 g L-1Figure 1 QuitoMax® effect on the germination percentage of corn seeds.
However, after 6 DAG, the desired physiological effect of QuitoMax® on germination was observed in treatments 2, 3 and 4, showing that the concentrations used were able to stimulate this process, reaching values above those of the control. Another important finding was that as the QuitoMax® concentration decreased, germination decreased; the best result was obtained in the concentration of 1 g L-1 corresponding to treatment 2.
In general, all the QuitoMax® treatments, including the 0.05 g L-1 (T4), which had the lowest number of germinated seeds, performed better than the control treatment, so it can be suggested that the germination rate of these seeds was slower than in treatments where chitosan was applied.
Some authors have suggested that chitosan increases germination in some crops, since it stimulates enzymes of secondary metabolism, such as chitinase, cellulase and B 1,3 glucanase 15,16. Additionally, treatment with chitosan was able to stimulate some events in the seed such as: hydration of proteins, subcellular structural changes, respiration, synthesis of macromolecules and cell elongation. All these processes allow the passage from a dehydrated embryo, in a resting state, with a barely detectable metabolism to one with an active metabolism that culminates in the growth of the embryonic axis.
The effect of QuitoMax® on the growth and development indicators of the corn studied are shown in Figure 2. In general, it can be seen that the concentration of 1 g L-1 achieved the greatest root length, the greatest stem length and the greatest plant height, but this concentration did not differ from the other treatments in which QuitoMax® was used, with the root length exception, but it did differ from the control treatment (T1).
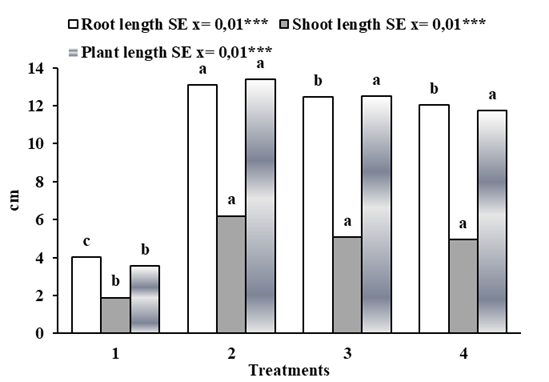 T1-Control,T2- Q:1 g L-1, T3- Q:0,5 g L-1, T4- Q:0,05 g L-1Means of treatments with same letters do not differ significantly with p<0.05 according to Duncan
T1-Control,T2- Q:1 g L-1, T3- Q:0,5 g L-1, T4- Q:0,05 g L-1Means of treatments with same letters do not differ significantly with p<0.05 according to DuncanFigure 2 QuitoMax® concentration effect on corn growth indicators and development up to the V2 stage of corn
Some authors have observed the growth stimulating effect of chitosan on cereal seeds, just as other researchers have observed an increase in stem size and diameter 17,18. This can be explained by the fact that chitosan favors the production of enzymes related to plant growth and development, such as cellulose, which promotes greater plant height 19.
It is necessary to emphasize that these results have a positive and practical impact in Cuban agriculture, because sowing is usually done after soaking and incubating the seeds for successive periods of 24 hours, although in Cuba, the soaking and resting time varies between 24 hours and 30 hours in summer and in winter up to 40 and 48 hours, respectively 20, which would accelerate this process with QuitoMax® use.
Effect of different application forms of QuitoMax® in early stages of the corn crop
When evaluating the effect of the different application forms of QuitoMax® on the root length of corn plants (Figure 3), differences were recorded between all of them and the treatment in which it was not applied (T1).
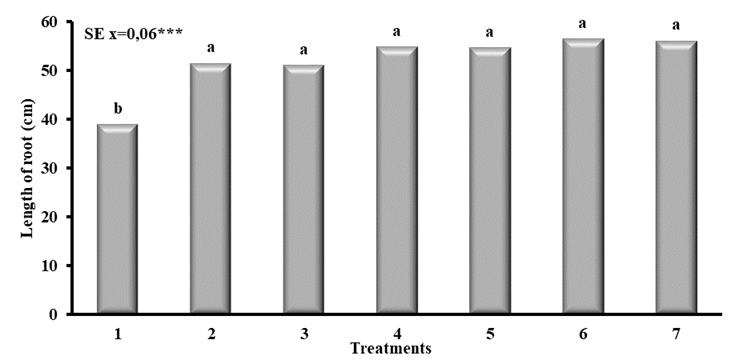 T1- Control, T2- Imbibition of seed 1 hour (1 g L-1), T3- Imbibition of seed 1 hour (0.5 g L-1), T4- Foliar spray (1 g L-1) (Dose 10 mL x Plant), T5- Foliar spray (0.5 g L-1) (Dose 10 mL x Plant), T6- Seed imbibition and foliar spray (1 g L-1), T7-Seed imbibition and foliar spray (0.5 g L-1)Means of treatments with equal letters do not differ significantly with p<0.05 according to Duncan
T1- Control, T2- Imbibition of seed 1 hour (1 g L-1), T3- Imbibition of seed 1 hour (0.5 g L-1), T4- Foliar spray (1 g L-1) (Dose 10 mL x Plant), T5- Foliar spray (0.5 g L-1) (Dose 10 mL x Plant), T6- Seed imbibition and foliar spray (1 g L-1), T7-Seed imbibition and foliar spray (0.5 g L-1)Means of treatments with equal letters do not differ significantly with p<0.05 according to DuncanFigure 3 Root length of corn plants treated with different application forms of QuitoMax®
It is noteworthy that in treatments where chitosan was applied, there were no significant differences among them; however, with chitosan application in the imbibition of seeds combined with foliar spraying (T6 and T7), superior root lengths were achieved compared to the independent applications (T2, T3, T4 and T5) and the control (T1).
The results of stem length evaluation are shown in Figure 4. It was observed that the plants where QuitoMax® was applied in different ways showed significantly superior results to those where the product was not applied (T1).
 T1- Control, T2- Imbibition of seed 1 hour (1 g L-1), T3- Imbibition of seed 1 hour (0.5 g L-1), T4- Foliar spray (1 g L-1) (Dose 10 mL x Plant), T5- Foliar spray (0.5 g L-1) (Dose 10 mL x plant), T6- Seed imbibition and foliar spray (1 g L-1), T7- Seed imbibition and foliar spray (0.5 g L-1)Means of treatments with same letters do not differ significantly with p<0.05 according to Duncan
T1- Control, T2- Imbibition of seed 1 hour (1 g L-1), T3- Imbibition of seed 1 hour (0.5 g L-1), T4- Foliar spray (1 g L-1) (Dose 10 mL x Plant), T5- Foliar spray (0.5 g L-1) (Dose 10 mL x plant), T6- Seed imbibition and foliar spray (1 g L-1), T7- Seed imbibition and foliar spray (0.5 g L-1)Means of treatments with same letters do not differ significantly with p<0.05 according to DuncanFigura 4 Stem length of corn plants treated with different application methods of QuitoMax®
When analyzing the effect of QuitoMax® on the height of corn plants (Figure 5), differences were recorded between treatments where chitosan was applied and the treatment where it was not applied (T1). It should be noted that among treatments in which chitosan was applied, best results, with significant differences with the rest of the treatments, were when seed imbibition was combined with foliar spraying, and within these when it was applied at a concentration of 1 g L-1 (T6).
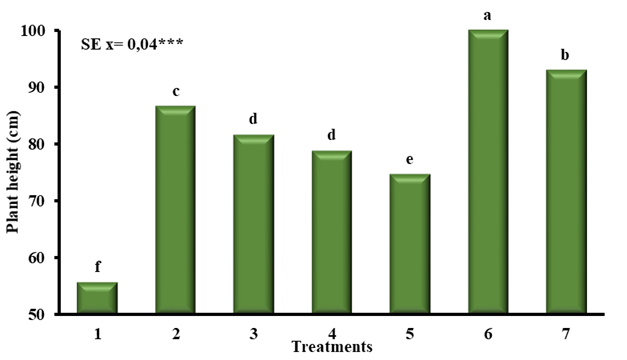 T1- Control, T2- Imbibition of seed 1 hour (1 g L-1), T3- Imbibition of seed 1 hour (0.5 g L-1), T4- Foliar spray (1 g L-1) (Dose 10 mL x Plant), T5- Foliar spray (0.5 g L-1) (Dose 10 mL x plant), T6- Seed imbibition and foliar spray (1 g L-1), T7- Seed imbibition and foliar spray (0.5 g L-1)Means of treatments with same letters do not differ significantly with p<0.05 according to Duncan
T1- Control, T2- Imbibition of seed 1 hour (1 g L-1), T3- Imbibition of seed 1 hour (0.5 g L-1), T4- Foliar spray (1 g L-1) (Dose 10 mL x Plant), T5- Foliar spray (0.5 g L-1) (Dose 10 mL x plant), T6- Seed imbibition and foliar spray (1 g L-1), T7- Seed imbibition and foliar spray (0.5 g L-1)Means of treatments with same letters do not differ significantly with p<0.05 according to DuncanFigure 5 Height of corn plants treated with different application methods of QuitoMax®
The response shown by plants treated with QuitoMax® in their growth and development agrees with that reported by other authors 21, when studying chitosan application effect on young maize (Zea mays L.) plants exposed to different types of stress. Similar responses in terms of growth and yield increase with QuitoMax® application were found when evaluating foliar application effect of this product on beans (Phaseolus vulgaris L.) 12.
Chitosan application effect on the dry mass of the root and aerial part of corn plants is shown in Figure 6. The behavior of the root dry mass (Figure 6A) showed that there were no statistical differences between means of the treatments where the product was applied and the control treatment (T1); however, it was found that the highest dry mass of the roots was obtained in plants to which seeds were embedded and then foliar sprayed with QuitoMax® at a dose of 1 g L-1.
In terms of root dry mass (Figure 6 B), the T6 treatment was the one that produced the most significant recovery impact, exceeding by about three times that found with T1. These favorable effects on plants where the different forms of chitosan application were used may be the result of the greater growth achieved by plants, which may enhance the increase in the content of essential elements 22.
 T1- Control, T2- Imbibition of seed 1 hour (1 g L-1), T3- Imbibition of seed 1 hour (0.5 g L-1), T4- Foliar spray (1 g L-1) (Dose 10 mL x Plant), T5- Foliar spray (0.5 g L-1) (Dose 10 mL x Plant), T6- Seed imbibition and foliar spray (1 g L-1), T7- Seed imbibition and foliar spray (0.5 g L-1)Means of treatments with equal letters do not differ significantly with p<0.05 according to Duncan
T1- Control, T2- Imbibition of seed 1 hour (1 g L-1), T3- Imbibition of seed 1 hour (0.5 g L-1), T4- Foliar spray (1 g L-1) (Dose 10 mL x Plant), T5- Foliar spray (0.5 g L-1) (Dose 10 mL x Plant), T6- Seed imbibition and foliar spray (1 g L-1), T7- Seed imbibition and foliar spray (0.5 g L-1)Means of treatments with equal letters do not differ significantly with p<0.05 according to DuncanFigura 6 Root dry mass (A) and aerial part (B) of corn plants treated with different application methods of QuitoMax®.
CONCLUSION
The application of QuitoMax® was effective in increasing the growth indicators of corn plants, being the combination of seed imbibition with foliar spraying at a concentration of 1 g L-1 the one with which the most important results are obtained. This suggests that the combination of the QuitoMax® application method is a recommendable option for corn cultivation.
BIBLIOGRAFÍA
1. Pérez-Madruga Y, Rosales-Jenquis PR, Menéndez DC, Falcón-Rodríguez A. Aplicación combinada de quitosano y HMA en el rendimiento de maíz. Cultivos Tropicales [Internet]. 2019;40(4). Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362019000400006&script=sci_arttext&tlng=pt1. [ Links ]
2. Herrera EMC, Toro M, Lopez D. Efecto de micorrizas nativas y fósforo en los rendimientos del maíz en Guárico, Venezuela. Temas Agrarios [Internet]. 2016;21(2):21-31. doi:10.21897/rta.v21i2.898 [ Links ]
3. Reyes GE, Cortés JD. Intensidad en el uso de fertilizantes en América Latina y el Caribe (2006-2012). Bioagro [Internet]. 2017;29(1):45-52. Available from: http://ve.scielo.org/scielo.php?pid=S1316-33612017000100005&script=sci_arttext&tlng=en3. [ Links ]
4. Torres-Rodríguez JA, Reyes-Pérez JJ, González-Gómez LG, Jiménez-Pizarro M, Boicet-Fabre T, Enríquez-Acosta EA, et al. Respuesta agronómica de dos variedades de maíz blanco (Zeas mays, L.) a la aplicación de QuitoMax, AZOFERT Y ECOMIC. Biotecnia [Internet]. 2018;20(1):3-7. Available from: https://biotecnia.unison.mx/index.php/biotecnia/article/view/5224. [ Links ]
5. Valdes YB. Manejo oportuno de las arvenses en sus relaciones interespecíficas con los cultivos del maíz (Zea mays L.) y del frijol (Phaseolus vulgaris L.) en un sistema sucesional [Internet] [Tesis de Doctorado]. [Instituto Nacional de Ciencias Agrícolas, Cuba]: Universidad Agraria de la Habana, Mayabeque, Cuba; 2017. 154 p. Available from: http://repositorio.geotech.cu/xmlui/bitstream/handle/1234/3659/Manejo%20de%20las%20arvenses%20en%20sus%20relaciones%20con%20los%20cultivos%20del%20ma%C3%ADz%20y%20del%20frijol.pdf?sequence=1&isAllowed=y5. [ Links ]
6. CITMA C. Enfrentamiento al cambio climático en la República de Cuba. Tarea Vida. La Habana: CITMATEL [Internet]. 2017; Available from: http://www.seapcuba.cult.cu/wp-content/uploads/2018/04/TAREA-VIDA-PLAN-DEL-ESTADO-PARA-EL-ENFRENTAMIENTO-AL-CAMBIO-CLIM%C3%81TICO.pdf6. [ Links ]
7. ONE. Anuario Estadístico de Cuba. Año 2018 [Internet]. Oficina Nacional de Estadística e Información, Sitio en Actualización. 2021 [cited 12/08/2021]. Available from: http://www.onei.gob.cu/node/138047. [ Links ]
8. Quintero Rodríguez E, Calero Hurtado A, Pérez Díaz Y, Enríquez Gómez L. Efecto de diferentes bioestimulantes en el rendimiento del frijol común. Centro Agrícola [Internet]. 2018;45(3):73-80. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0253-578520180003000738. [ Links ]
9. Hadwiger LA. Multiple effects of chitosan on plant systems: Solid science or hype. Plant science [Internet]. 2013;208:42-9. Available from: https://www.sciencedirect.com/science/article/abs/pii/S01689452130006309. [ Links ]
10. Hemantaranjan A, Deepmala K, Bharti S, Nishant Bhanu A. A Future Perspective in Crop Protection: Chitosan and its Oligosaccharides. Adv Plants Agric Res. 2014;1(1):1-6. Available from: https://d1wqtxts1xzle7.cloudfront.net/55553810/Chitosan-with-cover-page-v2.pdf?Expires=1630429646&Signature=V4i875cGA6-e-O2VzSXMFl8j9NrwTf5xno~9jl5luX1WiyAPMQJoEmll3c002gkZ2W0E6Q7gJ7tjTzkpWAqPKpdcj7585EshC7aQPIT1sz0A00tv1EZOXm5NHDHiPzxotR4QKakU75eCAA8qcHZIZFj-U8dPJETHh0xf5UGX~7J7n4TgfGHtG6VYHzdr1Hm0a1C6qleqxGkYOh44fO5Ex0sZ7XjH7Zp5JQWlIDVSnof38asJOdXegEvhzZw-8akWnJqj-SWD-Mvfrio7TIalUhxmfTDjy9JBCAywMUOy11oc6EH6vIg0pxepi0EkBBnR6XlSLujW-TZ3cQ8ARMVJxg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA10. [ Links ]
11. Ramírez MÁ, González P, Fagundo JR, Suarez M, Melian C, Rodríguez T, et al. Chitin Preparation by Demineralizing Deproteinized Lobster Shells with CO2 and a Cationite. Journal of Renewable Materials [Internet]. 2017;5(1):30-7. Available from: https://www.ingentaconnect.com/content/tsp/jrm/2017/00000005/00000001/art0000411. [ Links ]
12. Morales Guevara D, Dell Amico Rodríguez J, Jerez Mompié E, Hernández YD, Martín Martín R. Efecto del QuitoMax(r) en el crecimiento y rendimiento del frijol (Phaseolus vulgaris L.). Cultivos Tropicales [Internet]. 2016;37(1):142-7. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-5936201600010002012. [ Links ]
13. Morales Guevara D, Torres Hernández L, Jerez Mompié E, Falcón Rodríguez A, Amico Rodríguez JD. Efecto del Quitomax(r) en el crecimiento y rendimiento del cultivo de la papa (Solanum tuberosum L.). Cultivos Tropicales [Internet]. 2015;36(3):133-43. Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362015000300020&script=sci_arttext&tlng=en13. [ Links ]
14. HERNÁNDEZ J, PÉREZ J, BOSCH I, Castro SN. Clasificación de los suelos de Cuba. [Internet]. Cuba: Ediciones INCA; 2015. 93 p. Available from: https://isbn.cloud/isbn-upload-image/?isbn13=9789597023777 [ Links ]
15. Leubner-Metzger G. Functions and regulation of ß-1, 3-glucanases during seed germination, dormancy release and after-ripening. Seed Science Research [Internet]. 2003;13(1):17-34. Available from: https://www.cambridge.org/core/journals/seed-science-research/article/abs/functions-and-regulation-of-13glucanases-during-seed-germination-dormancy-release-and-afterripening/4280CBE92E6FE5DA15342CCCCC13EB9E15. [ Links ]
16. Rodríguez-Pedroso AT, Ramírez-Arrebato MA, Rivero-González D, Bosquez-Molina E, Barrera-Necha LL, Bautista-Baños S. Propiedades químico-estructurales y actividad biológica de la quitosana en microorganismos fitopatógenos. Revista Chapingo. Serie horticultura [Internet]. 2009 [cited 12/08/2021];15(3):307-17. Available from: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S1027-152X2009000500012&lng=es&nrm=iso&tlng=es16. [ Links ]
17. Martínez González L, Reyes Guerrero Y, Falcón Rodríguez A, Núñez Vázquez M. Efecto del tratamiento a las semillas con quitosana en el crecimiento de plántulas de arroz (Oryza sativa L.) cultivar INCA LP-5 en medio salino. Cultivos Tropicales [Internet]. 2015;36(1):143-50. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-5936201500010002017. [ Links ]
18. Pichyangkura R, Chadchawan S. Biostimulant activity of chitosan in horticulture. Scientia Horticulturae [Internet]. 2015;196:49-65. Available from: https://www.sciencedirect.com/science/article/abs/pii/S030442381530195318. [ Links ]
19. Pérez JJR, Enríquez-Acosta EA, Ramírez-Arrebato MÁ, Rodríguez-Pedroso AT, Lara-Capistrán L, Hernández-Montiel LG. Evaluation of the growth, yield and nutritional quality of pepper fruit with the application of Quitomax(r). International Journal of Agriculture and Natural Resources [Internet]. 2019;46(1):23-9. Available from: https://dialnet.unirioja.es/servlet/articulo?codigo=701936519. [ Links ]
20. Terry AE, Ruiz PJ, Falcón RA, Carrillo SY, Morales MH. Respuesta agronómica del cultivo de tomate al bioproducto Quitomax®20. . Cultivos Tropicales. 2017;38(1):7-13. Available from: https://www.cabdirect.org/cabdirect/abstract/2017320217920. [ Links ]
21. Lizárraga-Paulín EG, Torres-Pacheco I, Moreno-Martínez E, Miranda-Castro SP. Chitosan application in maize (Zea mays) to counteract the effects of abiotic stress at seedling level. African Journal of Biotechnology [Internet]. 2011;10(34):6439-46. doi:10.4314/ajb.v10i34 [ Links ]
22. Mondal M, Puteh AB, Dafader NC. Foliar application of Chitosan improved morphophysiological attributes and yield in summer tomato (Solanum lycopersicum). Pakistan Journal of Agricultural Sciences [Internet]. 2016;53(2):239-344. Available from: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1063.7518&rep=rep1&type=pdf22. [ Links ]
Received: September 09, 2020; Accepted: March 24, 2021











 texto en
texto en 


