Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cultivos Tropicales
versión impresa ISSN 0258-5936versión On-line ISSN 1819-4087
cultrop vol.42 no.4 La Habana oct.-dic. 2021 Epub 30-Dic-2021
Bibliographic review
Chitosan and its derivatives, natural polymers with potential for control of Pyriculariaoryzae (Cav.)
1Unidad Científico Tecnológica de Base “Los Palacios”, Instituto Nacional de Ciencias Agrícolas (INCA). Carretera La Francia km 1½, Los Palacios, Pinar del Río, Cuba. CP 22 900
2Centro de Desarrollo de Productos Bióticos, Instituto Politécnico Nacional, carretera Yautepec-Jojutla, km 6, San Isidro, CEPROBI 8, Yautepec, Morelos, México. CP 62731
3Departamento de Investigación y Posgrado en Alimentos, Universidad de Sonora, Blvd. Luis Encinas y Rosales s/n, Col. Centro, PO Box 1658, Hermosillo, Sonora, México. CP 83000
Chitosan and its derivatives are natural compounds that have potential in agriculture with respect to controlling one of rice diseases; blast (Pyriculariaoryzae) of great importance worldwide. In general, this disease is controlled with synthetic fungicides belonging to the benzimidazole group; however, their use has generated adverse results to the environment together with the low sensitivity of the fungus to them. This article provides a review of published research on chitosan, its physicochemical characteristics, general information on P. oryzaefungus, the chitosan fungicidal action and its derivatives in research carried out in vitro and in situ on this fungus and the possible mechanisms of action of this compound.
Key words: antimicrobials; biocompounds; action mechanisms; Magnaporthe; Oryzasativa
INTRODUCTION
Chitosan polysaccharide is a class of natural macromolecule that has an extremely bioactive tendency and it is derived from the exoskeleton of crustaceans such as lobsters, crabs and shrimps 1. Chitosan, a partially deacetylated polymer of chitin, has the ability to be biodegradable, biocompatible and non-toxic, which is why it is considered a very attractive compound. In agriculture, it is used to stimulate germination, modify soils, as a fungicidal agent and as an elicitor of defensive responses in plants, among others. Also in the food technology area, it is used in the elaboration of biodegradable films and antimicrobial packaging films 2,3.
This compound has demonstrated antifungal activity on different pathogens, among them Pyriculariaoryzae (Cav). This fungus produces the blast disease, which is of great importance in rice cultivation, causing great damage and is widely distributed throughout the world 4. Chitosan and its derivatives have been shown to act directly on the fungus inhibiting its mycelial growth and also stimulating the defense mechanisms in the rice crop and protecting the plant from attack by the same pathogen 5-8.
Chitosan. Chemical and physical characteristics
Chitosan is a polysaccharide obtained from chitin, the second most abundant polysaccharide in nature. It is a linear copolymer formed by residues of D-glucosamine units to a greater extent and N-acetyl D-glucosamine to a lesser extent, randomly distributed and joined by β 1,4 bonds. According to the International Union of Pure and Applied Chemistry (IUPAC), it is 2-amino 2-deoxy-D-glucopyranose (D-glucosamine GlcN) and 2-acetamide-2-deoxy-D-glucopyranose N-acetyl glucosamine 1. Both the content and sequence of these units will determine the physicochemical and biological properties of this polymer. Chitosan has a nitrogen (N) content greater than 7 and has a regular distribution of free amino groups, which can be protonated by certain acids, becoming positively charged and this gives it a polycationic character. This fact allows explaining some of its properties such as the ability to bind with negatively charged substances, such as lipids, proteins, colorants, among others; as well as flocculant, adherent and adsorbent, in addition to the typical reactions of amines 9. When the amino groups of chitosan bind to the acid amino acids of proteins, electrostatic interactions are produced that lead to cellular disorders in microorganisms.
This biopolymer possesses antimicrobial activity against a wide variety of microorganisms including fungi, algae and some bacteria. Its functionality and activity depends on its characteristics such as: molecular mass, acetylationdegree, host cell, presence of natural nutrients, chemical or nutritional composition of substrates and environmental conditions. In this sense, with respect to acetylation degree, it can be stated that the lower the acetylation degree, the higher the antimicrobial activity 10,11.
Pyriculariaoryzaecausal agent of rice blast or rice brusone
This plant pathogen is very efficient as it can reproduce sexually (teleomorph: Magnaporthegrisea Barr (It. Hebert) without Magnaportheoryzae) and asexually (anamorph: Pyriculariaoryzae). The Pyricularia/Magnaporthe Working Group has established under the auspices of the International Commission on Fungal Taxonomy (ICFT) the possibility of retaining the name Magnaporthe over Pyricularia. However, such conservation requires a change in the type species of the genus Magnaporthe and would cause numerous name changes for those species currently placed in Pyricularia12.
The asexually typed name Pyricularia is the correct name for the rice scorch fungus, which corresponds well with pathogenicity and ecological and evolutionary characteristics. Therefore, the name Pyriculariaoryzae should be used for the fungus that produces this disease. However, the synonym Magnaportheoryzae can continue to be referred to in publications as Pyriculariaoryzae (syn. Magnaportheoryzae). This practice will help to close a potential gap in the literature and knowledge of this important species 12. As for grisea and oryzae, they are very different species. Grisea is for strains of Digitaria and oryzae for rice strains, wheat and other grasses; although in the literature they are considered synonyms and the four names are used: Pyriculariaoryzae, Magnaportheoryzae, Pyriculariagrisea and Magnaporthegrisea.
Pyriculariaoryzae, known as blast or brusone is one of the most serious diseases of rice (Oryzasativa L.), which has caused significant losses in yields worldwide 13.
The blast distribution is worldwide since it is found in all agroecosystems of the tropics and temperate zones where commercial rice is grown. Piriculariosis generates large losses in grain production, both in rainfed and irrigated systems. This disease has been reported in at least 85 countries worldwide. It was discovered in Italy in 1560 and it was later found in China (1637), Japan (1760), United States (1960), and India (1913) 14. Blast has caused significant losses, for example: in India 90 % 15, in China 70 % 16, in Thailand only 1900 ha were affected in 1987, and in 1988 it increased to 490 000 ha, in Spain and in the Mediterranean area it has also caused damages 17. Mexico, on the other hand, has reported a drop in production of up to 30 % in rainfed crops. Cuba has been another country where this pathogen has caused damage, and when conditions are favorable, losses have increased up to 70 % 6.
Taxonomic classification, morphology and symptomatology of Pyriculariaoryzae
Taxonomic classification, morphology and symptomatology of Pyriculariaoryzae
The causal agent of blast is taxonomically classified in the class: Deuteromycetes, order: Moniliales, family: Dematiaceae, genus: Pyricularia, species: Pyriculariaoryzae. Pyriculariaoryzae has simple, partitioned, brownish conidiophores (Figure 1 A). The conidiophores are borne singly or in groups of three and carry conidia at their ends. The conidiophores are solitary or in groups of three and at their ends carry the conidia, which are hyaline, fusiform and divided by two equidistant septa.
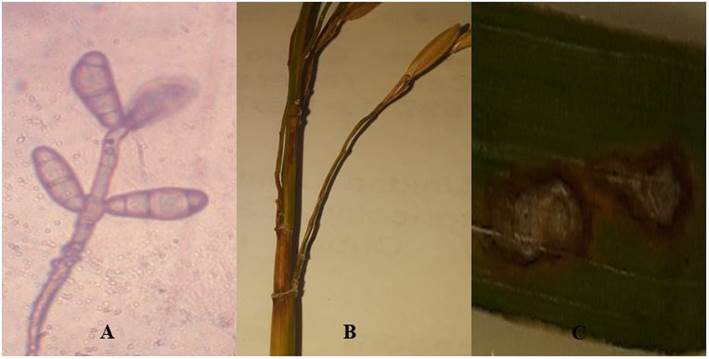
Authors' images
Figure 1 Conidia of Pyriculariaoryzae A), stem B) and leaf C) of rice infected by this fungus
It is a complex disease due to the pathogenic variability and the rapidity with which this fungus overcomes the resistance of the rice plant. The mycelium of the fungus produces a toxic substance known as pyricularin 16, which inhibits the growth of tissues and disorganizes them. It attacks the aerial parts of plant such as leaves, stems, nodes and spikes 18 (Figures 1 B, C). The fungus produces spots or lesions on leaves of elongated or elliptical to rhomboid shape and uniform brown color that later will change to a grayish color in the central part, a fact that indicates the sporulation of the fungus; although its size and color vary according to environmental conditions and the susceptibility of the varieties 19. Its optimum growth temperature is between 22-29 ºC and a high relative humidity around 90 %, which fully coincide with the climatic conditions of tropical countries. The presence of high concentrations of nitrogen in the water also favors the development of fungus and produces large quantities of spores. The spores arrive from the remains of the previous season's harvest or from weeds where the fungus has been lodged during the winter 20.
General strategies for the Pyriculariaoryzae control
Disease control is mainly based on the application of systemic chemical fungicides, including benzimidazole fungicides such as prochloraz, tebuconazole and propiconazole, among others. However, their use has had several disadvantages, since they cause contamination of the water table and adjacent bodies of water, generating harmful effects on various organisms. In addition, it is important to point out that these chemical fungicides are being damaged by the fungus, due to the emergence of populations with loss of sensitivity to their mode of action 21. Due to this situation, there is an increasing number of fungicide cycles per year to protect crops against this disease, which implies an increase in production costs and in negative effects of conventional fungicides on the environment, questioning their commercial use, being then a priority the search for alternatives that allow complementing the integrated management of diseases.
In vitro and in vivo activity of chitosan and its derivatives on Pyriculariaoryzae
The antimicrobial property of chitosan and its derivatives have received considerable attention in recent years due to the impending problem associated with synthetic chemical agents. These compounds have been shown to be fungicidal and fungistatic for the control of Botrytiscinerea, Aspergillusflavus, Aspergillusparasiticus, Drechsterasorokiana, Fusariumacuminatum, Fusariumgraminearum, Micronectriellanivalis, Rhizoctoniasolani, Alternariaalternata, Colletotrichumgloesporioides, Penicillumspp, Fusariumoxysporum and Bipolarisoryzae, among other fungi 22-26.
In this regard, the antifungal chitosan activity and its derivatives has been observed in different stages of fungal development, such as affecting the growth and development of the pathogen, sporulation, viability and germination of spores and the production of fungal virulence factors 23,27,28. Some authors have verified the fungicidal effect of these compounds at different concentrations and P. griseaisolates 5,7,29. Chitosan oligomers have also been shown to have a better inhibitory effect on this pathogen, achieving the minimum inhibitory concentration at a concentration higher than 2000 mg L-1 (30.
Studies carried out in the laboratory of Oligosaccharins of the National Institute of Agricultural Sciences (INCA) with P. grisea indicate that chitosan and its oligomers in the culture medium at a concentration of 1,000 mg L-1 and pH 5.6, totally inhibited the mycelial growth of this fungus 5. However, it is important to consider the pH of the resulting solution, which affects the positive charge of the amino groups, since in another test at pH 6 there was only a slight affectation of the fungus growth, although a total inhibition of sporulation was maintained 7.
Some research groups have begun to modify the chitosan molecule with the addition of hydrophobic groups to increase its biological activity against this pathogen. For example, N-sulfonated N-sulfobenzoyl chitosan 31, N,N,N. trimethyl chitosan 32 N,O-acyl chitosan 33, O-acyl chitosan 34,35, hydroxyethyl aryl chitosan 36, dimethylpiperazine chitosan 37, carboxymethyl chitosan 38, acyl urea thiourea chitosan 39, N-succinoyl chitosan 40) and N-heterocyclic chitosan 41. Researchers have noted that N-alkylation or N-arylation of chitosan with aromatic or aliphatic aldehydes effectively increased its antifungal activity on P. grisea42.
With the same methods and obtaining techniques, but using different types of aldehydes, other scientists 31, observed antifungal activity of 24 new chitosan derivatives (N-benzyl chitosan derivatives), which had a greater inhibitory effect than native chitosans on the growth and spore formation of P. grisea, with N-(m-nitrobenzyl) chitosan having the greatest effect at a concentration of 5 g L-1. These authors also observed that the most active derivative was N-(2,2diphenylethyl) chitosan, with a minimum inhibitory concentration of 0.3 g L-1 against this pathogen. On the other hand, great advances of chitosan and its oligomers on the direct control of rice diseases have been observed. Both chitin and chitosan have been shown to induce the accumulation of phytoalexin production in this case of momilactones A and momilactones B upon infection with P.grisea in rice leaves at a concentration of 10 µg mL-1 (43.
A group of researchers published the chitosan effect in stimulating defense responses in rice leaves 44. After treatment with 0.1 % chitosan, necrosis was clearly observed in the upper part of rice leaf. However, treating rice seedlings with 5 mg L-1 and inoculating them with Magnaporthegrisea 97-23-2D1 showed a better effect and control of the disease by more than 50 % 45. However, in 2007 it was evaluated, in semi-controlled conditions, where rice seeds were treated at different concentrations with two chitosans of different molecular weight 6. Eighteen days after seed germination, plants obtained were inoculated with spores of P. grisea, and the activity of enzymes related to defense such as PAL, glucanase, chitinase and chitosanase was determined, observing an increase in the activity of plants treated with elicitors, in relation to the control. In addition, no disease symptoms were observed in the highest concentration of both compounds used 6.
Currently, research is being conducted on the application of chitosan-based nanoparticles with antifungal activity and for the control of diseases such as pyriculariosis 8,46,47. On the other hand, researchers found that chitosan and silver (Ag) nanoparticles had a high antifungal activity on Pyriculariaoryzae at anAgconcentration (2 ppm) and chitosan (4000 ppm) 46. However, other scientists observed that applying 500 μl of a 0.1 % chitosan nanoparticle solution on rice leaves and 24 h later inoculated a spore suspension (1x105spores mL-1) of P. grisea8. After 10 days, no symptoms of the disease were observed; this could be due to the stimulation of some defense mechanism in leaves and controlled the infection 8.
Chitosan action mechanisms
Chitosan action mechanisms have not been fully established, although there are some hypotheses in this regard. In general, the various proposals to explain the antimicrobial activity of chitosan consider as a fundamental characteristic the polycationic nature of the molecule, which is given by the NH3+ groups of glucosamine, which confers important biological and physiological properties 48,49. In pH conditions, chitosan behaves as a linear polyelectrolyte, with a pK around 6.5, therefore at low pH the glucosamine residues are positively charged, due to the protonation of its amino residues, containing a high density of positive charges, which allows it to bind strongly to negatively charged surfaces 50. It is proposed that when the positive charge on the C-2 of the glucosamine monomer is below pH 6, chitosan is more soluble and has better antimicrobial activity than chitin 31,51.
Another proposed mechanism is the interaction between the positive charge of the chitosan molecule and the negative charge of the microbial membrane cells leading to the efflux of proteins and other intracellular constituents 31. The most abundant of the sphingolipids is mannosyldiinositrophosphate-ceramide (M(IP2)C), which has two negative charges. These negative charges corresponding to the plasma membrane (M(IP2)C) can bind to the amino groups of the glucosamine residues of chitosan. In other studies, it has been observed that chitosan forms transport channels for molecules in artificial lipid bilayers, which provides evidence that this compound can disorganize the cell membrane 50. A working group analyzed the chitosan action mode on fungal cells and observed two aspects: that chitosan permeabilizes the fungal plasma membrane and penetrates fungal cells, a process that is ATP-dependent, and also demonstrated that different cell types (conidium, germ tube and hyphae) exhibit different sensitivity to chitosan 52,53. In 2010, the same group of researchers demonstrated, through biological, biochemical, genetic and biophysical techniques, that the antifungal activity of chitosan depends on the fluidity of the fungal plasma membrane, which is determined by the composition of its polyunsaturated fatty acids, and this suggests a new strategy for antifungal therapy, involving treatments that increase the plasma membrane fluidity to make the fungus susceptible to biocompounds such as chitosan.
Chitosan also acts as a chelating agent that selectively binds trace metals and thereby inhibits toxin production and mycelial growth 54. It also activates some defense processes in host tissues 55 and inhibits several enzymes. The binding of chitosan to DNA and the inhibition of mRNA synthesis and protein synthesis 56, causing cellular and structural damage.
In the case of silver nanoparticles (Ag), it is based on the possibility that they adhere and penetrate the cell membrane 57, causing osmotic imbalance in spores, being very effective against Magnaporthegrisea58.
CONCLUSION
The reported literature shows that chitosan and its derivatives are capable of acting on P. oryzae, either directly, by inhibiting mycelial growth and spore production, or by inducing defense mechanisms in the rice plant, so these compounds could be used in agriculture, making it more sustainable. However, it is still convenient to deepen in other lines of research such as the evaluation of these compounds in field studies, the feasibility of developing commercial products based on this compound, focusing not only on control, but also on the possible action mechanisms by which these compounds act on the fungus and on the plant.
BIBLIOGRAFÍA
1. Harish Prashanth KV, Tharanathan RN. Chitin/chitosan: modifications and their unlimited application potential-an overview. Trends in Food Science and Technology. 2007;18(3):117-31. doi:doi:https//doi.org/10.1016/j.tifs.2006.10.022 [ Links ]
2. Ramos-García M de L, Bautista-Baños S, Barrera-Necha LL, Bosquez-Molina E, Alia-Tejacal I, Estrada-Carrillo M. Compuestos antimicrobianos adicionados en recubrimientos comestibles para uso en productos hortofrutícolas. Revista mexicana de fitopatología [Internet]. 2010;28(1):44-57. Available from: https://www.redalyc.org/articulo.oa?id=61214206005 [ Links ]
3. Kumar S, Mukherjee A, Dutta J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends in Food Science & Technology. 2020;97:196-209. doi:doi:https://doi.org/10.1016/j.tifs.2020.01.002 [ Links ]
4. Xing K, Zhu X, Peng X, Qin S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review. Agronomy for Sustainable Development [Internet]. 2015;35(2):569-88. doi:https://doi.org/10.1007/s13593-014-0252-3 [ Links ]
5. Rodríguez AT, Ramírez MA, Nápoles MC, Márquez R, Cárdenas RM. Antifungal activity of chitosan and one of its hydrolysates on Pyricularia grisea, Sacc. Fungus. Cultivos Tropicales [Internet]. 2003;24(2):85-8. Available from: https://www.redalyc.org/pdf/1932/193218174015.pdf [ Links ]
6. Rodríguez AT, Ramírez MA, Cárdenas RM, Hernández AN, Velázquez MG, Bautista S. Induction of defense response of Oryza sativa L. against Pyricularia grisea (Cooke) Sacc. by treating seeds with chitosan and hydrolyzed chitosan. Pesticide Biochemistry and Physiology [Internet]. 2007;89(3):206-15. doi:doi:10.1016/j.pestbp.2007.06.007 [ Links ]
7. Cárdenas RM, Ramírez MA, Rodríguez AT, González LM. Efecto de los derivados de quitina y su combinación con sulfato de cobre en el comportamiento del crecimiento micelial y esporulación de un aislamiento monospórico del hongo Pyricularia grisea Sacc. Cultivos Tropicales [Internet]. 2004;25(4):89-93. Available from: https://www.redalyc.org/pdf/1932/193225911012.pdf [ Links ]
8. Manikandan A, Sathiyabama M. Preparation of chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia grisea. International journal of biological macromolecules [Internet]. 2016;84:58-61. doi:doi: https:// doi.org/10. 1016/j.ijbiomac.2015.11.083 [ Links ]
9. Muzzarelli RAA. Enzymatic synthesis of chitin and chitosan. Occurrence of chitin. In: Muzzarelli RAA, editor. Chitin [Internet]. Pergamon; 1977 [cited 28/08/2021]. p. 5-44. doi:10.1016/B978-0-08-020367-6.50007-6 [ Links ]
10. Kong M, Chen X, Xue Y, Liu C, Yu L, Ji Q, et al. Preparation and antibacterial activity of chitosan microshperes in a solid dispersing system. Frontiers of Materials Science in China [Internet]. 2008;2(2):214-20. Available from: https://link.springer.com/content/pdf/10.1007/s11706-008-0036-2.pdf [ Links ]
11. Takahashi T, Imai M, Suzuki I, Sawai J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochemical Engineering Journal [Internet]. 2008;40(3):485-91. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1369703X08000430 [ Links ]
12. Zhang N, Luo J, Rossman AY, Aoki T, Chuma I, Crous PW, et al. Generic names in Magnaporthales. IMA fungus [Internet]. 2016;7(1):155-9. doi:doi:https://doi.org/10.5598/ imafungus.2016.07.01.09 [ Links ]
13. Lugo L, Jayaro Y, González Á, Borges O. Identification of sources of partial resistance to Pyricularia grisea in rice cultivars and experimental lines. Fitopatología Venezolana [Internet]. 2008;21(2):51-8. Available from: https://www.cabdirect.org/cabdirect/abstract/20093204367 [ Links ]
14. Rossman AY, Howard RJ, Valent B. Pyricularia grisea the correct name for the rice blast disease fungus. Mycologia [Internet]. 1990;82(4):509-12. Available from: https://www.tandfonline.com/doi/abs/10.1080/00275514.1990.12025916?journalCode=umyc20 [ Links ]
15. Manjunatha B, Krishnappa M. Morphological characterization of Pyricularia oryzae causing blast disease in rice (Oryza sativa L.) from different zones of Karnataka. Journal of Pharmacognosy and Phytochemistry [Internet]. 2019;8(3):3749-53. Available from: https://www.phytojournal.com/archives/2019/vol8issue3/PartBC/8-3-279-550.pdf [ Links ]
16. Ou SH. Rice diseases [Internet]. IRRI; 1985. Available from: https://books.google.es/books?hl=es&lr=&id=-k3mewv9nMoC&oi=fnd&pg=PR1&dq=Rice+Diseases&ots=Zmj_rBBc8i&sig=C413L03O2pB85A2EhfQhPD1KApU#v=onepage&q=Rice%20Diseases&f=false [ Links ]
17. Koutroubas SD, Katsantonis D, Ntanos DA, Lupotto E. Blast disease influence on agronomic and quality traits of rice varieties under Mediterranean conditions. Turkish Journal of Agriculture and forestry [Internet]. 2009;33(5):487-94. Available from: https://journals.tubitak.gov.tr/agriculture/abstract.htm?id=10484 [ Links ]
18. Kulmitra AK, Sahu N, Sahu MK, Kumar R, Kushram T, Sanath Kumar VB. Growth of Rice blast fungus Pyricularia oryzae (Cav.) on different solid and liquid media. International Journal of Current Microbiology and Applied Sciences [Internet]. 2017;6(6):1154-60. doi:https://doi.org/10.20546/ijcmas.2017.606.133 [ Links ]
19. Cárdenas RM, Pérez N, Cristo E, González MC, Fabré L. Estudio sobre el comportamiento de líneas y variedades de arroz (Oryza sativa Lin.) ante la infección por el hongo Pyricularia grisea Sacc. Cultivos Tropicales [Internet]. 2005;26(4):83-7. Available from: https://www.redalyc.org/pdf/1932/193216160012.pdf [ Links ]
20. Cárdenas RM, Polón CR, Pérez N, Cristo E, Mesa S, Fabré L, et al. Relación entre la incidencia de la piriculariosis (Pyricularia grisea Sacc.) del arroz (Oryza sativa Lin.) y diferentes variables climáticas en el Complejo Agroindustrial Arrocero Los Palacios. Cultivos Tropicales [Internet]. 2010;31(1):00-00. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-59362010000100002 [ Links ]
21. Manzo Sánchez G, Carrillo Madrigal H, Guzmán González S, Orozco Santos M. Análisis de la Sensibilidad in vitro de Mycosphaerella fijiensis, Agente Causal de la Sigatoka Negra del Banano a los Fungicidas Benomyl, Propiconazol y Azoxistrobin. Revista mexicana de fitopatología [Internet]. 2012 [cited 28/08/2021];30(1):81-5. Available from: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S0185-33092012000100008&lng=es&nrm=iso&tlng=es [ Links ]
22. Hua C, Li Y, Wang X, Kai K, Su M, Zhang D, et al. The effect of low and high molecular weight chitosan on the control of gray mold (Botrytis cinerea) on kiwifruit and host response. Scientia Horticulturae [Internet]. 2019;246:700-9. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0304423818308197 [ Links ]
23. Sánchez-Domínguez D, Ríos MY, Castillo-Ocampo P, Zavala-Padilla G, Ramos-García M, Bautista-Baños S. Cytological and biochemical changes induced by chitosan in the pathosystem Alternaria alternata-tomato. Pesticide biochemistry and physiology [Internet]. 2011;99(3):250-5. Available from: https://d1wqtxts1xzle7.cloudfront.net/44367734/Cytological_and_biochemical_changes_indu20160403-12579-18vumyq.pdf?1459730343=&response-content-disposition=inline%3B+filename%3DCytological_and_biochemical_changes_indu.pdf&Expires=1631769785&Signature=IGGgjxkuWLCyHKJnBKWJkxGt1dhC3jDsalPwe033Zh69KP~oMc9leISiFCA0WSiKUCxKb1K~RCJkP0URuU~09UxsZWRx6yjMBZ-~tCQYVGBeFq4iuV~0LuAXIeh1ysKZ53HVKZb0Z2fVsYsTRC~FMcGsjqZbRwVPDq1mjM7VE43h3RvolAkQ4LLzRWs1KSSscUcUHoy-8cVMHx~zmJFU7DCf~ZGlzwERIsr7TgSW2w7xT7vzMf-OaiiFMda~v241ukzc5Vd6HBXZUxr1J16f266xhUCV5w6uDBnm4X3MHMaFFjIaf9XPSJdfnmDnR8TAzi11jPKfVHXjjuNPU0Y8Zg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA [ Links ]
24. Cortés-Higareda M, de Lorena Ramos-García M, Correa-Pacheco ZN, Del Río-García JC, Bautista-Baños S. Nanostructured chitosan/propolis formulations: characterization and effect on the growth of Aspergillus flavus and production of aflatoxins. Heliyon [Internet]. 2019;5(5):e01776. Available from: https://www.sciencedirect.com/science/article/pii/S2405844019309806 [ Links ]
25. Sahariah P, Masson M. Antimicrobial chitosan and chitosan derivatives: a review of the structure-activity relationship. Biomacromolecules [Internet]. 2017;18(11):3846-68. Available from: https://pubs.acs.org/doi/abs/10.1021/acs.biomac.7b01058 [ Links ]
26. Rodríguez Pedroso AT, Plascencia Jatomea M, Bautista Baños S, Cortez Rocha MO, Ramírez Arrebato MÁ. Actividad antifúngica in vitro de quitosanos sobre Bipolaris oryzae patógeno del arroz. Acta Agronómica [Internet]. 2016;65(1):98-103. Available from: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-28122016000100014 [ Links ]
27. Zivkovic S, Stevanovic M, Durovic S, Ristic D, Stosic S. Antifungal activity of chitosan against Alternaria alternata and Colletotrichum gloeosporioides. Pesticidi i fitomedicina [Internet]. 2018;33(3-4):197-204. Available from: https://plantarum.izbis.bg.ac.rs/bitstream/handle/123456789/543/541.pdf?sequence=1 [ Links ]
28. Badawy ME, Rabea EI. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. International Journal of Carbohydrate Chemistry [Internet]. 2011. doi:https://doi.org/10.1155/2011/460381 [ Links ]
29. Rabea EI, Badawy ME, Rogge TM, Stevens CV, Höfte M, Steurbaut W, et al. Insecticidal and fungicidal activity of new synthesized chitosan derivatives. Pest Management Science [Internet]. 2005;61(10):951-60. doi:https://doi.org/10.1002/ps.1085 [ Links ]
30. Xu J, Zhao X, Han X, Du Y. Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pesticide Biochemistry and Physiology. 2007;87(3):220-8. [ Links ]
31. Chen C-S, Liau W-Y, Tsai G-J. Antibacterial effects of N-sulfonated and N-sulfobenzoyl chitosan and application to oyster preservation. Journal of Food Protection [Internet]. 1998;61(9):1124-8. doi:https://doi.org/10.4315/0362-028X-61.9.1124 [ Links ]
32. Jia Z, Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydrate research [Internet]. 2001;333(1):1-6. doi:https://doi.org/10.1016/S0008-6215(01)00112-4 [ Links ]
33. Sashiwa H, Kawasaki N, Nakayama A, Muraki E, Yamamoto N, Zhu H, et al. Chemical modification of chitosan. Synthesis of organosoluble, palladium adsorbable and biodegradable chitosan derivatives toward the chemical plating on plastics. Biomacromolecules [Internet]. 2002;3(5):1120-5. doi:https://doi.org/10.1021/bm0200478 [ Links ]
34. Badawy ME, Rabea EI, Rogge TM, Stevens CV, Steurbaut W, Höfte M, et al. Fungicidal and insecticidal activity of O-acyl chitosan derivatives. Polymer bulletin. 2005;54(4):279-89. doi:https://doi.org/10.1007/s00289-005-0396-z [ Links ]
35. Badawy M, Rabea E, Steurbaut W, Rogge T, Stevens C, Smagghe G, et al. Fungicidal activity of some O-acyl chitosan derivatives against grey mould Botrytis cinerea and rice leaf blast Pyricularia grisea. Communications in agricultural and applied biological sciences [Internet]. 2005;70(3):215-8. Available from: https://europepmc.org/article/med/16637180 [ Links ]
36. Ma G, Yang D, Tan H, Wu Q, Nie J. Preparation and characterization of N-alkylated chitosan derivatives. Journal of applied polymer science [Internet]. 2008;109(2):1093-8. doi:https://doi.org/10.1002/app.28223 [ Links ]
37. Másson M, Holappa J, Hjálmarsdóttir M, Rúnarsson ÖV, Nevalainen T, Järvinen T. Antimicrobial activity of piperazine derivatives of chitosan. Carbohydrate polymers [Internet]. 2008;74(3):566-71. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0144861708001720 [ Links ]
38. Seyfarth F, Schliemann S, Elsner P, Hipler U-C. Antifungal effect of high-and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. International Journal of Pharmaceutics [Internet]. 2008;353(1-2):139-48. Available from: https://pubmed.ncbi.nlm.nih.gov/18164151/ [ Links ]
39. Zhong Z, Xing R, Liu S, Wang L, Cai S, Li P. Synthesis of acyl thiourea derivatives of chitosan and their antimicrobial activities in vitro. Carbohydrate Research [Internet]. 2008;343(3):566-70. Available from: https://www.researchgate.net/profile/Shengbao-Cai/publication/5765859_Synthesis_of_acyl_thiourea_derivatives_of_chitosan_and_their_antimicrobial_activities_in_vitro/links/5fd7458745851553a0b591d5/Synthesis-of-acyl-thiourea-derivatives-of-chitosan-and-their-antimicrobial-activities-in-vitro.pdf [ Links ]
40. Tikhonov VE, Stepnova EA, Babak VG, Yamskov IA, Palma-Guerrero J, Jansson H-B, et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2 (3)-(dodec-2-enyl) succinoyl/-derivatives. Carbohydrate polymers. 2006;64(1):66-72. [ Links ]
41. Stössel P, Leuba JL. Effect of chitosan, chitin and some aminosugars on growth of various soilborne phytopathogenic fungi. Journal of Phytopathology [Internet]. 1984;111(1):82-90. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1439-0434.1984.tb04244.x [ Links ]
42. Badawy ME, Rabea EI. Characterization and antimicrobial activity of water-soluble N-(4-carboxybutyroyl) chitosans against some plant pathogenic bacteria and fungi. Carbohydrate polymers [Internet]. 2012;87(1):250-6. Available from: http://damanhour.edu.eg/pdf/researches/1-s2.0-S014486171100645X-main.pdf [ Links ]
43. Shimizu T, Jikumaru Y, Okada A, Okada K, Koga J, Umemura K, et al. Effects of a bile acid elicitor, cholic acid, on the biosynthesis of diterpenoid phytoalexins in suspension-cultured rice cells. Phytochemistry [Internet]. 2008;69(4):973-81. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0031942207006097?via%3Dihub [ Links ]
44. Agrawal GK, Rakwal R, Tamogami S, Yonekura M, Kubo A, Saji H. Chitosan activates defense/stress response (s) in the leaves of Oryza sativa seedlings. Plant Physiology and Biochemistry. 2002;40(12):1061-9. [ Links ]
45. Lin W, Hu X, Zhang W, Rogers WJ, Cai W. Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. Journal of Plant Physiology [Internet]. 2005;162(8):937-44. Available from: http://sippe.ac.cn/gh/papers/caiweiming/Lin2005.pdf [ Links ]
46. Pham DC, Nguyen TH, Ngoc UTP, Le NTT, Tran TV, Nguyen DH. Preparation, characterization and antifungal properties of chitosan-silver nanoparticles synergize fungicide against Pyricularia oryzae. Journal of nanoscience and nanotechnology [Internet]. 2018;18(8):5299-305. Available from: https://www.researchgate.net/profile/Dinh-Chuong-Pham/publication/321308729_Preparation_Characterization_and_Antifungal_Properties_of_Chitosan-Silver_Nanoparticles_Synergize_Fungicide_Against_Pyricularia_oryzae/links/5a35486245851532e82f1da0/Preparation-Characterization-and-Antifungal-Properties-of-Chitosan-Silver-Nanoparticles-Synergize-Fungicide-Against-Pyricularia-oryzae.pdf [ Links ]
47. Nguyen TH, Thi TV, Nguyen T-T, Le TD, Vo DMH, Nguyen DH, et al. Investigation of chitosan nanoparticles loaded with protocatechuic acid (PCA) for the resistance of Pyricularia oryzae fungus against rice blast. Polymers. 2019;11(1):177. [ Links ]
48. Je J-Y, Kim S-K. Antimicrobial action of novel chitin derivative. Biochimica et Biophysica Acta (BBA)-General Subjects [Internet]. 2006;1760(1):104-9. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0304416505003028?via%3Dihub [ Links ]
49. El Hadrami A, Adam LR, El Hadrami I, Daayf F. Chitosan in plant protection. Marine drugs. 2010;8(4):968-87. [ Links ]
50. Zakrzewska A, Boorsma A, Brul S, Hellingwerf KJ, Klis FM. Transcriptional response of Saccharomyces cerevisiae to the plasma membrane-perturbing compound chitosan. Eukaryotic Cell [Internet]. 2005;4(4):703-15. Available from: https://journals.asm.org/doi/pdf/10.1128/EC.4.4.703-715.2005 [ Links ]
51. Velásquez CL. Some potentialities of chitin and chitosan for uses related to agriculture in Latin America. Revista Científica UDO Agrícola. [Internet]. 2008;8(1):1-22. [ Links ]
52. Palma-Guerrero J, Huang I-C, Jansson H-B, Salinas J, Lopez-Llorca LV, Read ND. Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Fungal Genetics and Biology [Internet]. 2009;46(8):585-94. Available from: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.458.5391&rep=rep1&type=pdf [ Links ]
53. López Jiménez JÁ, Palma Guerrero J, Pérez Berná AJ, Huang IC, Jansson HB, Salinas J, et al. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Molecular Microbiology [Internet]. 2010;75(4) Available from: https://digitum.um.es/digitum/bitstream/10201/38147/1/Membrane%20fluidity%20determines%20sensitivity.pdf [ Links ]
54. Cuero RG, Duffus E, Osuji G, Pettit R. Aflatoxin control in preharvest maize: effects of chitosan and two microbial agents. The Journal of Agricultural Science [Internet]. 1991;117(2):165-9. Available from: https://www.cambridge.org/core/journals/journal-of-agricultural-science/article/abs/aflatoxin-control-in-preharvest-maize-effects-of-chitosan-and-two-microbial-agents/BD8D14313D49649D683B711CE3F4DDE4 [ Links ]
55. El Ghaouth A, Arul J, Asselin A, Benhamou N. Antifungal activity of chitosan on post-harvest pathogens: induction of morphological and cytological alterations in Rhizopus stolonifer. Mycological research [Internet]. 1992;96(9):769-79. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0953756209804474?via%3Dihub [ Links ]
56. Sudarshan NR, Hoover DG, Knorr D. Antibacterial action of chitosan. Food Biotechnology [Internet]. 1992;6(3):257-72. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0953756209804474?via%3Dihub [ Links ]
57. Avila-Quezada GD, Espino-Solis GP. Silver nanoparticles offer effective control of pathogenic bacteria in a wide range of food products. Pathogenic Bacteria [Internet]. 2019; Available from: https://www.intechopen.com/chapters/69239 [ Links ]
58. Jo Y-K, Kim BH, Jung G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant disease [Internet]. 2009;93(10):1037-43. Available from: https://apsjournals.apsnet.org/doi/abs/10.1094/PDIS-93-10-1037 [ Links ]
Received: July 02, 2020; Accepted: March 24, 2021











 texto en
texto en 



