Introduction
In December 2019, a new coronavirus emerged in China and caused an acute respiratory disease known as coronavirus disease 2019 (COVID-19).1 The etiological agent of COVID-19, the SARS-CoV-2 rapidly spread to others countries producing the most devastating pandemic of recent times. As of June 14, 2020, the virus had infected 7 759 691 persons in 185 countries, 5,3% of whom had died.
Because of the rapid increase in number of cases and uncontrolled and vast spread worldwide, the World Health Organization (WHO) declared SARS-CoV-2 a pandemic.2 Rapid identification of the etiology and sharing of the genetic sequence of the virus, followed by international collaborative efforts initiated because of emergence of SARS-CoV-2, led to rapid availability of real-time reverse transcription quantitative PCR (RT-PCR) diagnostic to confirm the viral infection.3
As in many other transmissible diseases, serological techniques to detect IgM and IgG antibodies could be of importance for serological diagnostic and seroprevalence studies. Many serological commercial kits have been developed and used in testing patient specimens for COVID‐19 by Chinese CDC, US CDC, and other private companies. Some of them are already certified by accredited international organizations
Today, there is an urgent need for a rapid, simple to use, sensitive, and accurate serological tests to quickly identify infected patients of SARS‐CoV‐2 to prevent virus transmission and to assure timely treatment of patients. Additionally, test for massive screening and probably for travelers testing are also needs. We show the results of a fast evaluation of seven SARS-CoV-2 rapid IgM/IgG tests for the detection of IgG/IgM to SARS-CoV-2 both in serum and whole blood.4,5,6,7
The fundamental objective of this work is to evaluate seven commercial systems for the rapid detection of antibodies to determine their sensitivity, specificity and robustness in our conditions for use by the National Health System.
Methods
Diagnostic tests. Seven lateral flow chromatographic immunoassays (Wondfo, Lungene, Deangel, Realy Tech, Deep Blue, Orient Gene and Assut Europe) for the qualitative detection of IgM/IgG antibodies to SARS-CoV-2 were evaluated at the Institute of Tropical Medicine “Pedro Kourí” (IPK) of Havana, Cuba. Manufacturer instructions were followed in each case. Table 1 shows the main characteristics of the evaluated tests.
All tests were performed on an immunochromatographic strip contained in a cassette on which the sample is discharged and then a buffer is added. The evaluated systems have a control line for validity. Positivity is reflected with a different line for each immunoglobulin (IgM and IgG), except in the Wondfo system that detects both in a single one line. In each kit evaluation at list two observers validate the diagnostic.
Table 1 General characteristics of the lateral flow chromatographic immunoassays for IgM/IgG to Coronavirus/COVID-19 evaluated
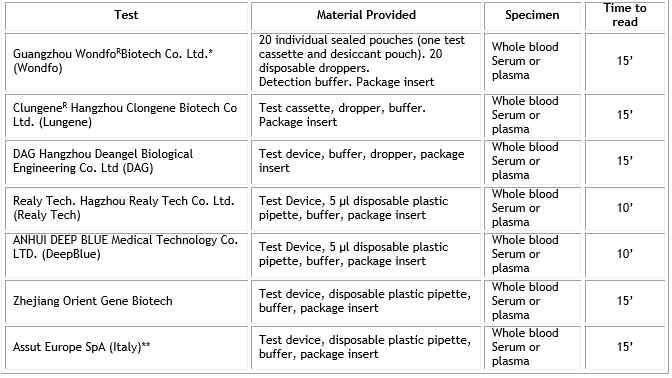
*Detect IgM/IgG antibodies in one line.
** Except this test all the rest were developed in China.
Samples
Negative control samples include
a) Whole blood obtained by venipuncture (N= 100); from healthy volunteers work at the general areas of IPK without contact relation with patients at the hospital or samples at the laboratories of the institution.
b) 25 serum samples from IPK archives received through the national laboratory viral surveillance previous to 2020, with a confirmed diagnostic of measles, dengue, hepatitis and other respiratory viruses (Influenza A and seasonal coronavirus HKU1).
Positive samples
Positive control panel includes samples (N= 44) collected from patients symptomatic and asymptomatic hospitalized at IPK with a confirmed diagnostic of COVID-19 by RT-PCR in nasopharyngeal swabs (Modular Wuhan CoV E and RdRP genes, Roche).
Ethical issues
The study was conducted in compliance with the Declaration of Helsinki and using Good Laboratory Practices. Informed consents were obtained from patients and volunteers. The specimens tested from IPK serum archives were residual samples received through the national surveillance of viral infectious diseases in Cuba before 2020. The study maintained always the anonymity and integrity of the patient and volunteers.
Statistical analysis
The test data were collected and analyzed. Sensitivity, Specificity, Concordance, predictive value of a positive result (PPV) and predictive value of a negative result (NPV) and Kappa coefficient were calculated for each kit evaluated using an android application for viral diagnosis.8 Sensitivity was defined as the proportion of patients correctly identified as having SARS-CoV-2 infections, as initially diagnosed using nucleic acid detection of SARS-CoV-2 in respiratory samples. Specificity was defined as the proportion of SARS-CoV-2 immune naïve study participants accurately identified as negative for SARS-CoV-2.
Results
Evaluation of the seven rapid test for detection of SARS-CoV-2 IgM or/and IgG
We evaluated seven rapid tests for IgM or/and IgG detection, to determine their analytical performance and its utility in the current epidemiological Cuban situation. Of the serum samples from IPK archives with a confirmed diagnostic of measles, dengue, hepatitis and other respiratory viruses (Influenza A and seasonal coronavirus), none of them were positive in the rapid tests evaluated.
The rapid tests were evaluated using serum and whole blood samples collected from RT-PCR-positive cases with SARS-CoV-2 and for control. Table 2 shows the overall Sensitivity, Specificity, NPV and PPV, Concordance and Kappa coefficient of the seven rapid tests compared with the RT PCR in case of positive samples. In case of Lungene test is shown the results for IgG detection, as the IgM was detected only in the 21.7% of the samples tested. Figure 1 summarizes the sensitivity and specificity of the total antibodies detected by the seven rapid test.
Table 2 Analytical performance of seven rapid tests for IgM or/and IgG anti- SARS CoV-2 infection
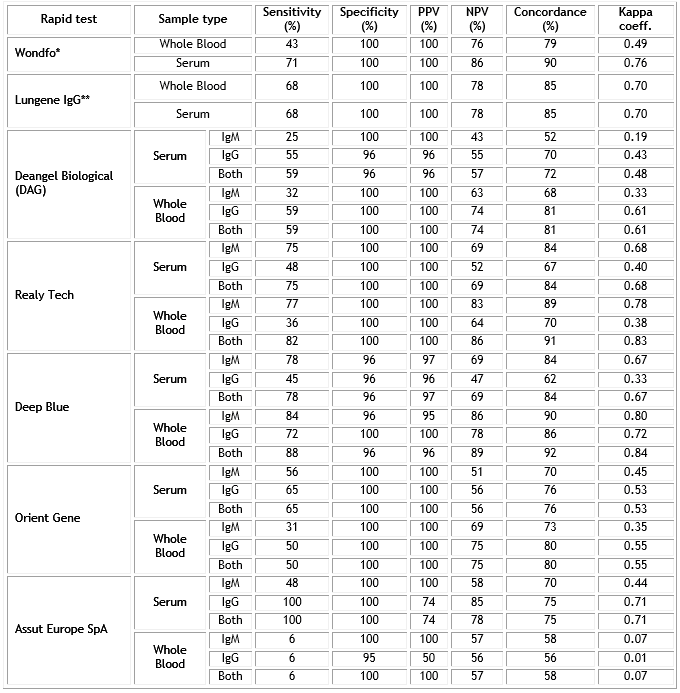
*Detect total Abs.
**The system did not detect any IgM in positive cases (serum or whole blood).
Overall sensitivity figures ranges from 25 to 88%, showing Realy Tech and Deep Blue rapid tests the better results. Similarly, overall specificity figures were almost 100% in all tests with the exception of Deangel Biological and deep Blue rapid test (96%). Wondfo, Realy Tech and Deep Blue rapid tests showed the highest concordance with SARS-CoV-2 RT-PCR (over 90%).
Realy Tech and Deep Blue tests showed the higher overall sensitivity (82 and 88% respectively) in whole blood with a good specificity and concordance while Wondfo and Deangel tests showed a lower sensitivity (43 and 59% respectively). Realy Tech and Deep Blue tests also showed in whole blood a sensitivity of 77 and 84% respectively for IgM detection exclusively but a low sensitivity for IgG detection in case of Really Tech (36%). In whole blood Deep Blue test had the better results in IgM and IgG detection than the other four tests.
In serum, Realy Tech and Deep Blue tests followed by Wondfo test showed the highest figures of overall sensitivity (75, 78 and 71% respectively). In general, the highest sensitivity of Realy Tech and Deep Blue tests both in serum and whole blood was mainly based on IgM detection. Taken IgM and IgG results together, these tests showed the higher sensitivity.
Finally, as a measure of agreement between the two techniques, we found almost perfect agreement (0.81-1.00) again in Realy Tech and Deep Blue rapid tests.
Antibody detection relative to the stages of the disease
As the proportion of patients with positive virus specific IgG and/or IgM reached 90% approximately 14 days after symptoms onset (DASO), we analyze the positivity of Realy Tech and Deep Blue tests in serum samples collected in patients at early, intermediate and at late stage of the disease. Table 3 displays the results.
Table 3 Positivity (in percent) of Realy Tech and Deep Blue rapid tests in serum collected from patients according to days after symptoms onset
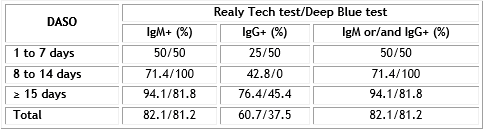
The IgM positive rate according Realy Tech or Deep Blue was rising from 50% in early stage to 71.4 or 100% in intermediate stage and to 94.1 or 81.8% in late stage of the disease respectively. The IgG positive rate in the confirmed patients was 25% in early, to 42.8% in intermediate stage and increasing to 76.4% in late stage according to Realy Tech test; on the contrary in case of Deep Blue test the IgG positivity did not increase over time. Combining both parameters the overall positivity remains similar to IgM detection.
Antibody detection relative to the clinical picture of the patients
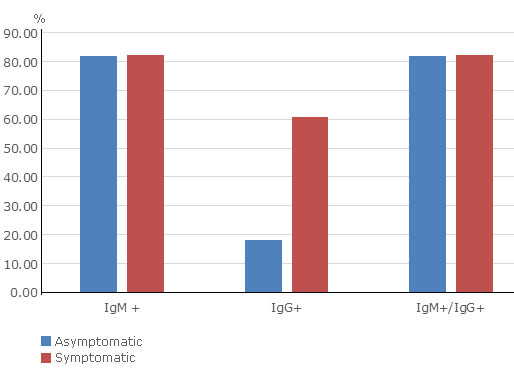
To investigate whether rapid tests could help to identify infected SARS-CoV-2 individuals without symptoms, we assessed the positivity of the Realy Tech rapid test in serum collected from asymptomatic infected individuals and symptomatic patients (Fig. 2). The rapid test was positive in the 81.8 and 82.1% of asymptomatic individuals and symptomatic patients respectively included in the study. Higher figures of IgM rather than IgG antibody detection were observed.
Discussion
More than ever, countries need supporting diagnostic tools for the diagnostic and surveillance with adequate figures of sensitivity, specificity, rapidity as well as low cost. Antibody IgM/IgG detection, a routine diagnostic practice for many illnesses, has become a challenge for COVID-19. From one side countries need of a serological tool for a rapid and early detection of infected individuals, on the other side, there is not sufficient information on the kinetic of the humoral immune response. Consequently, rapid serological tests need to be careful evaluated.9,10
In the evaluation of immunochromatographic systems, the Realy Tech and Deep Blue tests showed the best sensitivity and specificity figures. While specificity was not a problem in kit analysis, sensitivity was below 80% in many of the systems. Although we recognize that one of the limitations of our study may be in the low number of samples analyzed. The results obtained suggest that the tests corresponding to the Realy Tech and Deep Blue systems are capable of detecting IgM both in serum and in whole blood with adequate sensitivity and specificity. It was observed that cross-reactivity to related diseases does not seem to be a problem, including other seasonal coronavirus that were discriminated by the systems, although it is necessary to see other diseases than in evaluations carried out in other studies, such as malaria or leptospirosis, they cause false positives as well as collagen diseases that, especially in the detection of IgM, can also give positivity.
Normally, when a virus invades the body, the IgM response occurs first against the virus; and with the progression of the disease, IgG antibodies begin to be produced and gradually become detectable. In the case of SARS CoV-2 infection, data have shown that positive IgM and/or IgG rates are extremely low in the first five days after the initial onset of symptoms, because antibodies are not produced in the most patients in this early stage, and they increase rapidly as the disease progresses.11,12,13,14
Unfortunately the number of samples analyzed in this study in relation to each of the stages of infection does not allow a more in-depth comparative study on the kinetics of antibodies; even more so this does not was the goal of the study in the first place. However, our results will allow a better interpretation of the rapid tests used.
Although serology in the case of COVID-19 cannot be used as a diagnostic technique because have limited utility in the diagnosis of acute covid-19 it allows epidemiological surveillance, to know the immune status of populations and in certain populations to carry out with a more economically accessible test, a screening where the disease is suspected and before a notable increase in IgM positive cases, assumes recent circulation and then apply diagnostic techniques such as PCR or antigen detection.
Even with limitations in the evaluations, or to identify acute cases and for the identification of asymptomatic and pre-symptomatic patients. Serological tests are essential to analyze the immune response against the infection to carry out their epidemiological characterization, of SARS-CoV-2 and potentially inform individual risk of future disease and the study of potential vaccines.