Introduction
Acute disseminated encephalomyelitis (ADEM) usually is a monophasic inflammatory demyelinating disease of the central nervous system (CNS), predominantly affecting the white matter. In rare cases, it can evolve in a biphasic manner, making it difficult to establish a differential diagnosis with other demyelinating diseases, such as Multiple Sclerosis.1,2) ADEM is more prevalent in children, who have a more favorable prognosis, but it can also affect adults.3,4 Most cases of ADEM occur following vaccination or viral infections. The manifestations include neurological changes, which can vary from lethargy and altered level of consciousness to seizures and coma.5 Infections such as zika fever, dengue fever, or chikungunya, which are very common in the Brazilian health context, can also precede the occurrence of ADEM.3,6,7
Although the pathophysiology of ADEM is not fully understood, it is possible that the rupture of the blood-brain barrier in the inflammatory phase in the presence of infections and the autoimmune response to myelin proteins and neuronal cells are involved in this process. Because of the probable autoimmune etiology of ADEM, it is important to confirm the presence of an infectious agent in the CNS, since most autoimmune manifestations are triggered by the overstimulation of the immune system.3,8 In addition, imaging studies, especially magnetic resonance imaging (MRI) of the brain, are extremely important for the diagnosis of ADEM.3
Studies and knowledge about the development of neuropathies after infection by the dengue, zika, and chikungunya viruses have evolved in recent years. However, there are still few published studies related to the occurrence of ADEM after infection with these arboviruses.3,6,7
Thus, the aim of the present study was to carry out a bibliographical survey about the clinical manifestations and radiological findings of ADEM after infection with dengue, zika, and chikungunya viruses, to expand the knowledge about this association.
Methods
This work is a bibliographic research of qualitative nature, which has as main objectives the subjective analysis of the studies and the description of the complexity of the subject addressed.9
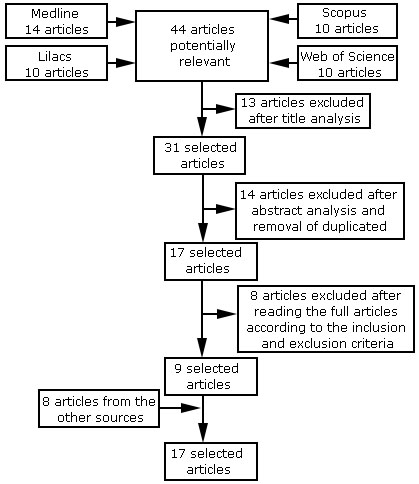
For the collection of information, an electronic search of articles was carried out in the months of June and July 2020 in four databases: MEDLINE, LILACS, Web of Science, and Scopus, using a combination of the terms “Zika virus”, “Dengue virus”, “Chikungunya virus” and “Encephalomyelitis, Acute Disseminated”. Articles published between 2010 and 2020 and written in Portuguese, English, and Spanish were included. The initial search yielded 44 articles, 35 of which were excluded after title and abstract analysis, duplicate removal, and full-text reading. In addition, eight publications obtained from references of the previous selected articles were added, totaling 17 articles selected to compose the results and discussion of this review (Fig.).
Aiming at a more precise study about the association of ADEM with arboviruses, the following inclusion criteria were adopted: studies with case reports that established correlations between neurological manifestations of ADEM, characteristic radiological findings, and infection with Zika, dengue, or chikungunya viruses. Communications and experience reports were excluded. In the set of analysis techniques, the analysis by topics was used, which is the division of the text into units, where the analysis becomes effective on the condition of applying direct and simple interpretations. It is important to consider the individual risk of bias in each study.
Results
The number of clinical cases reported in the literature about ADEM associated with arboviruses is relatively small. In this context, to facilitate the understanding of the main clinical and radiological manifestations of this condition, the main findings of the selected articles are summarized in Table below.
Table Summary of data extracted from the 17 selected articles.
| Study | Authors and year of publication | Location | Year of collection | Study patients | Virus | Age and sex | Clinical manifestations of viral disease | Neurological manifestations | Mean time of onset of neurological symptoms since the onset of the disease | Evolution post-treatment | Changes on MRI/CT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Brito Ferreira |
Pernambuco, Brazil | 2014-2015 | 1 | Zika | 53, F | Fever, rash, itching, asthenia, anorexia, retro-orbital pain, conjunctival hyperemia, edema in hands, knees, and feet | Alteration of the level of consciousness, bilateral visual deficit, motor and sensory deficit | 7 days | Motor sequelae in lower limbs | MRI showed confluent and bilateral T2 hyperintense lesions in the periventricular white matter and in the thoracic spine |
| 2 | Alves-Leon |
Rio de Janeiro, Brazil | 2015 | 1 | Zika | 35, F | Not informed | Somnolence, mental confusion, walking disturbance, diplopia, right-side hemiparesis, dysphasia | Not informed | Mild dysphagia and pyramidal signs without motor symptoms | MRI showed multiple supratentorial tumefactive lesions surrounded by vasogenic edema. Lesions with peripheral gadolinium enhancement, pattern of “open ring” enhancement |
| 3 | Galliez |
Rio de Janeiro, Brazil | 2016 | 1 | Zika | 26, M | Malaise, fever | Paraparesis, lower limb paraesthesia, urinary retention | 1 day | Flaccid paraparesis | MRI of the brain showed multiple patchy areas of T2/FLAIR hyperintensity in both white matter hemispheres, affecting deep and subcortical regions. MRI also showed a remarkable high signal on FLAIR/T2 in both medial cerebellar peduncles, and an elongated cranial-caudal hyperintensity area after pyramidal tract location |
| 4 | Mancera-Páez |
Cucutá, Colombia | 2016 | 1 | Zika | 24, F | Vomiting, fever, myalgia, epigastric pain | Alteration of the level of consciousness, urinary retention, bilateral lower limb weakness | 30 days | Urinary and bowel incontinence, complete loss of sensation in lower limbs | MRI of the of the brain and spinal cord showed multiple T2 hyperintense, nonenhancing lesions located in the periventricular white matter and subcortical areas, and multiple T2 hyperintense, nonenhancing lesions in the medulla oblongata, cervical, and thoracic spinal cord |
| 5 | Roth |
New York, United States of America | 2016 | 1 | Zika | 18, F | Malaise, nausea, nonpruritic macular rash of the trunk, legs, palms, and soles of the feet | Impairment of fine touch and temperature from the T6 dermatome, numbness in lower limbs | 47 days | Not informed | MRI of the cervical, thoracic, and lumbar spine showed multiple T2 hyperintense, nonenhancing lesions in the cervical and thoracic cords. Brain MRI was significant for multifocal subcortical and callosal T2/FLAIR hyperintense lesions, some of which with faint enhancement and restricted diffusion |
| 6 | Gupta |
New Delhi, India | 2013 | 1 | Dengue | 26, F | High fever, vomiting, arthralgia, myalgia | Altered consciousness (comatose), morbilliform, maculopapular rash involving arms, legs, and torso | 6 days | Not informed | MRI of the brain showed T2 hyperintensities involving right temporal neocortex, cerebellum, bilateral symmetrical periventricular and subcortical white matter of frontal and parietal lobe and callosal-septal interface |
| 7 | Martinez, |
Havana, Cuba | Not informed | 1 | Dengue | 50, F | Fever, headache, myalgia, erythematous and pruritic lesions | Mild irritability, difficulty concentrating, mental confusion, left-side hemiparesis | 15 days | Complete neurological recovery | MRI of the brain showed multiple T2 hyperintense lesions at the hemispheric and periventricular level in the parietal and temporal lobes bilaterally |
| 8 | Bhat (2010) | Punjab, India | 2010 | 1 | Dengue | 14, M | Fever, headache, vomiting | Tonic-clonic seizures followed by loss of consciousness | 5 days | Not informed | MRI of the brain showed symmetrical T2/FLAIR hyperintensities involving bilateral cerebellar hemispheres, bilateral middle cerebellar peduncles, brain stem, bilateral thalami, hypothalamus and periventricular deep and subcortical white matter |
| 9 | Bhoi |
Lucknow, India | 2013 | 1 | Dengue | 16, M | Moderate fever, chills, myalgia, headache, vomiting, erythematous maculopapular rash | Change in the level of consciousness, neck stiffness, somnolence, right-side hemiparesis | 5 days | Improvement of right-side hemiparesis | MRI of the brain showed increased signal intensity in corona radiata bilaterally and right peritrigonal white matter. T2/FLAIR hyperintense lesion in the left basal ganglia |
| 10 | Gera C, Uttam G (2010) | Punjab, India | 2005 | 1 | Dengue | 27, M | Fever, myalgia, vomiting | Tonic-clonic seizures, coma | Not informed | Complete neurological recovery | MRI of the brain showed T2/FLAIR hyperintensities in the periventricular and subcortical white matter bilaterally in the frontal and parietal lobes and also in the left temporal lobe. T2 images showed small areas of hemorrhage involving both thalami and the cerebellum |
| 11 | Sundaram |
Hyderabad, India | Not informed | 1 | Dengue | 27, M | High-grade fever, chills, headache, vomiting, hematochezia | Obtunded level of consciousness, mild neck stiffness | Not informed | Death due to complications (ARDS, acute kidney injury, and metabolic acidosis). | MRI of the brain showed T1 hypointense lesions involving bilateral hippocampi, thalami, cerebellar hemispheres, and posterior part of the pons. These lesions were hyperintense on T2-weighted images |
| 12 | Chowdhury |
Daca, Bangladesh | Not informed | 1 | Dengue | 13, F | Fever, low backache, headache, retro-orbital pain | Seizures, altered level of consciousness, myoclonus | 4 days | Recovery of the level of consciousness, headache, dysarthria, ataxia | MRI of the brain showed T2/FLAIR hyperintense lesions in the bilateral thalamic region due to demyelination |
| 13 | Gala |
Mumbai, India | Not informed | 1 | Dengue | 8, F | Fever, hemoptysis | Tonic-clonic seizures, somnolence, aggressive behavior, hallucinations | 12 days | Complete neurological recovery | CT scan of the brain suggested bilateral frontal and occipital lobe hypodensities in the white matter |
| 14 | Karoli |
Uttar Pradesh, India | Not informed | 1 | Dengue | 32, F | High grade fever with chills, myalgias, arthralgia, abdominal pain, vomiting | Altered level of consciousness, tonic-clonic seizures | 14 days | Complete neurological recovery | MRI of the brain showed FLAIR hyperintense lesions |
| 15 | Viswanathana |
Malaysia | Not informed | 2 | Dengue | Case 1: 61, F; Case 2: 30, M | Case 1: Fever, lethargy, arthralgia, myalgia, diarrhea; Case 2: Fever, myalgia, arthralgia, nausea, headache, vomiting, diarrhea | Case 1: Urinary incontinence, altered mental status, coma; Case 2: altered level of consciousness, ophthalmoplegia, quadriparesis followed by seizures | Case 1: not informed; Case 2: 18 days | Case 1: Gradual cognitive improvement over 2 months. Post discharge, patient was lost to follow-up; Case 2: Complete cognitive recovery. Able to stand and walk with support | Case 1: MRI of the brain done within the first week of illness showed no abnormalities. Repeat MRI of the brain showed parieto-occipital subcortical periventricular hyperintense lesions and T1 hypointensities. Case 2: MRI of the brain showed T2/FLAIR bilateral hyperintensities involving both thalami, brainstem, and corpus callosum |
| 16 | Carvalho |
Rio de Janeiro, Brazil | 2017 | 1 | Chikungunya | 35, M | 1-day unmeasured fever, headache, myalgia, diffuse maculopapular rash with no pruritus | Subtle left motor deficit, midline and appendicular ataxia on the left side, multidirectional nystagmus in both eyes | 34 days | Complete neurological recovery | MRI of the brain showed multiple subcortical lesions, hyperintense white matter in T2/FLAIR, T1 hypointensity, increased hypointensity in the center of T2, discrete peripheral enhancement without perilesional edema, and lesions in the cerebellum and midbrain |
| 17 | Teixeira |
Fortaleza, Brazil | 2017 | 1 | Chikungunya | 16, F | High grade fever, rash, arthralgia in hands, wrists, and knees | Diffuse pain in lower limbs, paresis, unstable gait | Not informed | Ataxia in lower limbs, apalestesis, hyperreflexia in lower limbs associated with persistent clonus, urinary incontinence | MRI of the brain showed T2/FLAIR hyperintense lesion in the corpus callosum, subcortical white matter, and medulla without restriction to diffusion. MRI of the spine revealed extensive T2 hyperintense area, predominantly posterior from C1 to C7 and central in the thoracic spine |
Source: Prepared by the author
Adem associated with Zika Virus (ZIKV)
Zika virus (ZIKV) targets human brain cells, causing immune activation. In contrast with asymptomatic patients, reports of neurological disorders are usually severe.1 In the present bibliographic research, five cases of ADEM associated with Zika fever were found. The cases occurred between the years 2014 and 2016, and three cases were in Brazil (Table).
In the first Brazilian case, the patient, a 53-year-old female diagnosed with Zika fever, presented with alteration of the level of consciousness, bilateral visual deficit, and motor and sensory deficit in the lower limbs, 7 days after viral symptoms. Patient’s MRI examination showed confluent and bilateral T2 hyperintense lesions in the periventricular white matter and in the thoracic spine, and the patient was diagnosed with ADEM.10
Another case in Brazil was that of a 35-year-old female patient with a previous diagnosis of multiple sclerosis (MS) in 2012. It is known that multiple sclerosis (MS) is an important differential diagnosis of ADEM, since the first demyelinating event of ADEM might also represent the first manifestation of MS.11 Basically, ADEM is a polysymptomatic encephalopathy, which has a duration of approximately 3 months, and MS is an isolated monofocal or polyfocal syndrome, without encephalopathy.12 In addition, the clinical method is extremely important to differentiate these two conditions, correlating manifestations with a particular patient's history. In 2015, this patient presented with acute encephalopathy initiated with somnolence, mental confusion, walking disturbance, and diplopia, later revealing consciousness impairment, dysphasia, right-side hemiparesis, inter-nuclear ophthalmic paresis, and cerebellar ataxia. The MRI examination showed multiple supratentorial tumefactive lesions with peripheral gadolinium enhancement, surrounded by vasogenic edema. Some lesions followed the pattern of “open ring” enhancement, and the patient was diagnosed with ADEM. ZIKV was confirmed by positive anti-ZIKV IgM and IgG antibodies in the serum and urine. IgG was detected in the cerebrospinal fluid (CSF) sample. The patient underwent brain biopsy in the left frontal region and ZIKV was detected by immunohistochemical assays. ZIKV RNA was detected on brain biopsy. The hypothesis of Zika virus antigens in brain tissue was raised by the severe immune response, indicating a possible correlation between the virus and severe neurological manifestations. ADEM diagnosis was suspected in this case due to the acute appearance of her neurological symptoms and the epidemiological data, highlighting again the importance of the clinical method, which allowed the medical team to think of the diagnosis of ADEM even with a previous history of MS.1
Another Brazilian report is the case of a 26-year-old male patient who, 1 day after having fever and malaise, developed paraparesis, lower limb paraesthesia, and urinary retention. On the 3rd day he had tetraparesis but had normal brain computerized tomography (CT) scan. Acyclovir and ceftriaxone were started. On day 7, he had reduced level of alertness and neck stiffness. The patient followed simple verbal commands, but did not respond properly to the examiner’s questions. He had bilateral facial palsy, with preserved corneal reflexes. Muscle strength was grade 0 in the lower limbs and 2 in the upper limbs. Deep tendon reflexes were increased in the upper limbs. Patellar reflexes were normal, and ankle jerks were absent. On day 7, CT scan remained normal. Urine and CSF collected 1 week after the initial symptoms were positive for ZIKV reverse transcriptase-polymerase chain reaction (RT-PCR). After 3 weeks, brain MRI showed multiple patchy areas of T2/FLAIR hyperintensity at both white matter hemispheres, affecting deep and subcortical regions. Both medial cerebellar peduncles and an elongated cranial-caudal hyperintensity area after pyramidal tract location were compatible with encephalomyelitis.13
A study published in Colombia reported the case of a 24-year-old female patient that presented with vomiting, fever (around 40ºC), myalgia and severe epigastric pain, diagnosed with ZIKV infection by RT-PCR. The patient developed urinary retention and bilateral lower limb weakness with foot drop. The weakness ascended and she developed flaccid quadriplegia, became confused, hypoxemic, and required respiratory support. MRI of the of the brain and spinal cord showed multiple T2 hyperintense, nonenhancing lesions located in the periventricular white matter and subcortical areas, in the medulla oblongata and cervical and thoracic spinal cord. The patient’s condition and the tests performed confirmed the presence of ZIKV associated with ADEM and Guillain-Barré syndrome.6
In the United States, an 18-year-old female patient developed malaise, nausea, and non-pruritic macular rash of the trunk, legs, palms, and soles of the feet, 2 weeks after a trip to the Dominican Republic. The patient was diagnosed with Zika fever by PCR. Six weeks after the onset of the symptoms, she awoke with numbness in both legs extending from both knees to both feet and extending proximally to the trunk above the umbilicus. Her neurological examination was remarkable for impairment of fine touch and temperature from the T6 dermatome extending distally and in both hands. Babinski sign was positive on the left foot. MRI of the cervical, thoracic, and lumbar spine with and without contrast showed multiple T2 hyperintense, nonenhancing lesions in the cervical and thoracic cords. MRI of the brain was significant for multifocal subcortical and callosal T2/FLAIR hyperintense lesions, some of which with faint enhancement and restricted diffusion, consistent with ADEM.14
Adem associated with Dengue Virus
The dengue virus (DENV) has spread rapidly around the world in recent years, and has become a global public health problem. DENV has four serotypes (DENV 1-4) and, as with the Zika and chikungunya viruses, is transmitted to humans by the bite of the female mosquito Aedes aegypti.15 In the present study, ADEM associated with dengue virus infection was reported in ten cases between the years 2010 and 2019.
Gupta et al. followed a 26-year-old female patient that presented with high fever, vomiting, arthralgia and myalgia, with confirmed dengue virus infection. On the 6th day of the fever onset, as the first neurological manifestation, she developed altered level of consciousness. Physical examination revealed a morbilliform, maculopapular rash involving arms, legs, and torso. On neurological assessment, she was comatose (Glasgow coma scale score 8/15), moving all limbs equally on painful stimuli, and had normal pupil size, absent meningeal signs, and a positive Babinski sign. MRI of the brain revealed T2 hyperintensities involving right temporal neocortex, cerebellum, bilateral symmetrical periventricular and subcortical white matter of frontal and parietal lobe and callosal-septal interface, characteristic of ADEM.16
Martinez et al. published a case report in which a 50-year-old female patient, with a history of arterial hypertension, reported, for a period of 5 days, fever accompanied by headache, myalgia, and erythematous papular and pruritic lesions on the body. Two weeks after the onset of symptoms, the patient had mild irritability, difficulty concentrating on some activities of daily living, and mental confusion. She was admitted to the emergency room and underwent a CT scan of the brain, which showed no pathological changes. In the neurological examination, a change in consciousness and left-side hemiparesis of moderate intensity were noted. MRI of the brain showed multiple T2 hyperintense lesions with an inflammatory aspect in the parietal and temporal lobes bilaterally, suggestive of ADEM.5
Another study reported a male patient who presented with moderate and intermittent fever (not associated with chills), intermittent and diffuse headache, and non-bilious vomiting for 6 days prior to admission. In addition, he manifested a change in the level of consciousness 5 days after the onset of symptoms and tonic-clonic seizures, which lasted 15 minutes and were followed by loss of consciousness. On general physical examination, the patient had multiple petechial spots over the trunk, and there was swelling over the eyelids. Neurological examination revealed neck stiffness, intermittent decerebrate posturing, brisk deep tendon reflexes, hypertonia in all the four limbs, and sluggishly reacting pupils to light. Since the patient had fever, petechial spots, low platelets, and a positive result for dengue virus, the initial diagnosis was hemorrhagic dengue fever. However, MRI of the brain showed symmetrical T2/FLAIR hyperintensities involving bilateral cerebellar hemispheres, bilateral middle cerebellar peduncles, brain stem, bilateral thalami, hypothalamus and periventricular deep and subcortical white matter, suggesting ADEM.17
Bhoi et al. reported the case of a 16-year-old male patient who had moderate fever associated with chills, non-throbbing holocranial headache, vomiting, and myalgia for 12 days. On the 5th day after the onset of symptoms, the patient developed erythematous maculopapular and petechial rashes over the entire body, accompanied by a progressive change in the level of consciousness. The patient had no history of seizures, otorrhea, rhinorrhea, or recent vaccination. During the physical exam, he was drowsy and had neck stiffness. His serum dengue IgM was positive. MRI of the brain showed T2/FLAIR hyperintensity in genu and splenium of corpus callosum and right centrum semiovale. The patient developed pneumonia and sepsis, for which he was mechanically ventilated and started on antibiotics. Despite the improvement in the general condition after treatment, hemiparesis persisted, and, after 20 days, another MRI of the brain was performed, showing increased signal intensity in corona radiata bilaterally, right peritrigonal white matter and a heterogeneous T2/FLAIR hyperintense lesion in the left basal ganglia. In addition, restricted diffusion was detected in the basal ganglia. The left basal ganglia lesion showed areas of blooming on gradient-echo (GRE) sequence suggesting hemorrhage. Post-contrast T1 sequence revealed ring enhancement in the left basal ganglia lesion. Because of these findings, the patient was diagnosed with ADEM.7
An Indian study published in 2010 also addressed a case of ADEM following infection with dengue virus. A 27-year-old male patient presented with history of fever, myalgia, and vomiting for 5 days, in addition to two episodes of tonic-clonic seizures. On admission, he was comatose. MRI of the brain showed T2/FLAIR hyperintensities in the periventricular and subcortical white matter bilaterally in the frontal and parietal lobes and also in the left temporal lobe. T2 images showed small areas of hemorrhage involving both thalami and the cerebellum. The thalami, pons, and cerebellar hemispheres showed restricted diffusion. The patient was diagnosed with ADEM.18
Another Indian case published in 2010 reported a 27-year-old male patient who presented with high-grade fever associated with chills and rigors of 5 days’ duration, headache and vomiting of 3 days’ duration, and altered level of consciousness of 1 day’s duration. CNS examination showed obtunded level of consciousness and mild neck stiffness. Serum IgG and IgM dengue antibodies were positive. MRI of the brain showed T1 hypointense lesions involving bilateral hippocampi, thalami, cerebellar hemispheres, and posterior part of the pons. These lesions were hyperintense on T2-weighted images. The patient developed myalgia, jaundice, conjunctival hemorrhage, hematuria, oliguria, and shortness of breath. He also developed adult respiratory distress syndrome (ARDS), acute kidney injury, and metabolic acidosis. Patient rapidly deteriorated and died. At autopsy, the diagnosis was ADEM with multi-organ failure.19
Chowdhury et al. described the case of a 13-year-old female patient who presented with high and continuous fever for 5 days and generalized seizures for 1 day. The patient also had severe low backache, headache, and retro-orbital pain. On examination on the day of admission, she was restless, febrile, and had generalized erythematous macular rash. She developed generalized seizures preceded by vomiting and loss of consciousness. Later on, the patient developed involuntary movement in the form of myoclonus. On the 2nd day after admission, she developed hematemesis and melena. Neurological examination showed that the patient was restless and unconscious. Glasgow Coma Scale (GCS) was 6/15. Pupils were mid-dilated, symmetrical with sluggish reaction to light. The diagnosis of ADEM was confirmed by MRI of the brain, which revealed T2/FLAIR hyperintense signal changes in the bilateral thalamic region, parietal white matter, and cerebellum due to demyelination.20
In 2012, Gala et al. published the case report of an 8-year-old female patient who presented with history of fever for 6 days and one episode of hemoptysis, which was followed by shortness of breath. On admission, the patient was cyanosed, restless with labored breathing and therefore, was intubated and ventilated. Dengue IgG was positive whereas dengue IgM was negative. On the 5th day of hospital admission, she was weaned from the ventilator. Twelve hours post-extubation, she had two episodes of tonic-clonic seizures. Next day, the patient had multiple episodes of seizures, altered level of consciousness, somnolence, aggressive behavior, and hallucinations. A CT scan of the brain was performed and revealed bilateral frontal and occipital lobe hypodensities in the white matter, which suggested demyelination. In view of the clinical picture and CT findings, the patient was diagnosed with ADEM.21
A study by Karoli et al. reported the case of a 32-year-old female patient who presented with high grade fever with chills, myalgias and arthralgia along with abdominal pain and occasional vomiting for 5-6 days. On physical examination, she was febrile and had tachycardia. The serum IgM antibody to dengue virus was positive. She was treated and her condition improved. However, about two weeks later, she returned to the emergency room in state of altered level of consciousness with history of two episodes of generalized tonic-clonic seizures. The patient had normal vitals and general examination except mild pallor. Neurological examination showed that she was drowsy and disoriented. MRI of the brain revealed patchy areas of FLAIR hyperintense signals. The patient was diagnosed with ADEM associated with dengue virus infection.4
Finally, the 10th study reported two clinical cases. The first case was that of a 61-year-old Indian woman who presented with a 3-day history of fever, lethargy, arthralgia, myalgia, and diarrhea. On the 4th day, she developed sudden onset of urinary incontinence with altered mental status, generalized body weakness, and coma. Pupils were reactive. Serum IgG and IgM dengue antibodies were positive. MRI of the brain done within the first week of illness showed no abnormalities. However, repeat MRI of the brain showed T2/FLAIR fronto-temporal, parieto-occipital subcortical periventricular hyperintense lesions and T1 hypointensities. The patient was diagnosed with ADEM. The second case was that of a 30-year-old male patient who presented with history of fever, myalgia, arthralgia, poor oral intake of 2 weeks’ duration with nausea, headache, vomiting and diarrhea. On the 4th day of hospital admission, the patient had altered level of consciousness, became restless and agitated and developed ophthalmoplegia and quadriparesis followed by seizures. The serum IgM antibody to dengue virus was positive. MRI of the brain showed bilateral T2/FLAIR hyperintensities involving both thalami, brainstem, and corpus callosum. The patient was diagnosed with ADEM following dengue virus infection.22
Adem associated with Chikungunya Virus
The Chikungunya Virus (CHIKV) is transmitted by the bite of infected female Aedes aegypti and Aedes albopictus mosquitoes. CHIKV was isolated in the early 1950s and disseminated to several countries, including Brazil. The clinical manifestations of chikungunya include fever, arthralgia, muscle pain, headache, nausea, and fatigue. In certain cases, there may be the presence of neurological complications, which are related to a less favorable prognosis. Regarding CHIKV infection, the present bibliographic research found two cases in the literature, both of which occurred in Brazil between 2019 and 2020.
Carvalho et al. reported the case of a 35-year-old male patient who presented with significant visual impairment in both eyes initiated 10 days earlier. The patient reported recent yellow fever immunization, but no antibody response for yellow fever was detected, possibly ruling out its relationship with ADEM. Neurological examination showed a subtle left motor deficit, midline and appendicular ataxia on the left side, and multidirectional nystagmus in both eyes. MRI of the brain revealed multiple subcortical lesions, hyperintense white matter in T2/FLAIR, T1 hypointensity, increased hypointensity in the center of T2, discrete peripheral enhancement without perilesional edema, and lesions in the cerebellum and midbrain, compatible with ADEM. There was a positive reactivity for IgM against CHIKV. The patient presented with an abrupt onset of visual impairment, discrete motor and balance alterations, a multidirectional nystagmus, characteristic MRI findings, and two potential provoking previous infections for ADEM, of which the most likely was the relationship with chikungunya.3
Teixeira et al. reported the case of a 16-year-old female patient who presented with progressive diffuse pain in the lower limbs, associated with paresis and unstable gait. She also reported a 20-day history of high grade fever, rash, and severe arthralgia in her hands, wrists, and knees. Neurological examination revealed quadriparesis presenting full-range movement of the upper and lower limbs only with gravity eliminated, without any changes in reflexes or sensitivity. MRI of the brain showed T2/FLAIR hyperintense lesion in the corpus callosum, subcortical white matter, and medulla without restriction to diffusion. MRI of the spine revealed extensive T2 hyperintense area, predominantly posterior from C1 to C7 and central in the thoracic spine, compatible with ADEM findings. Chikungunya IgM was positive in serum and in CSF.23
Discussion
Considering the arboviruses Zika fever, dengue fever, and chikungunya, most cases of ADEM reported in the researched literature were associated with dengue fever, representing 61.1% of the total number of individuals cited in the present study. ZIKV infection was the second most common infection, representing 27.8% of the patients mentioned in this study. The cases of ADEM associated with infection by chikungunya virus made up 11.1% of this total.
The majority of patients who had ADEM as a complication after arbovirus infection were female (61.1%), and the age group affected ranged from 8 years to 61 years, as can be seen in Table. According to Gray et al., ADEM is a disease that shows no significant difference in terms of the ratio of men to women affected. The study also states that ADEM related to other causes is a disease of young people, but its clinical presentation tends to appear before the age of 20, especially in children aged 5 to 8 years, different from what was observed regarding ADEM associated specifically with arboviruses.24
ADEM is an acute inflammatory demyelinating encephalopathy.25 According to Pohl et al., there is usually a prodromal period of the disease, which can include symptoms such as fever, malaise, irritability, somnolence, nausea, and vomiting. Thus, it is clear that based on the case reports selected and summarized in Table, there are similarities regarding this precursor stage of the disease. In this context, 88.9% of patients had fever and 50% had episodes of nausea and/or vomiting. In addition, other initial clinical manifestations were noted in these patients with ADEM associated with arboviruses, such as myalgia, present in 50% of the selected cases; headache, present in 38.9%; rash, present in 33.3%, and arthralgia, present in 27.8% of patients. Other symptoms were also observed, but in smaller proportions, such as diarrhea, chills, asthenia, abdominal pain, and retro-orbital pain.26
Neurological symptoms of ADEM typically occur between 2 days to 4 weeks after a viral infection or post-vaccination.27 In the present study, the mean time of onset of neurological symptoms after the onset of symptoms of infection was around 15 days, which is consistent with the current literature.
According to Karoli et al., the hallmark of ADEM clinical features is the development of focal or multifocal neurological disorder. Initial clinical feature include encephalopathy and focal or multiple neurological signs due to involvement of basal ganglia, thalamus, cranial nerves, or spinal cord. Regarding these neurological symptoms, the clinical presentation of ADEM can be diverse and is determined by the location and severity of the lesions in the CNS. Neurological symptoms mainly include changes in mental status, ranging from lethargy to coma, as well as unilateral or bilateral pyramidal signs (60%-95%), acute hemiplegia (76%), ataxia (18% to 65%), cranial nerve palsy (22%-45%), visual loss due to optic neuritis (7%-23%), seizures (13%-35%), spinal cord involvement (24%), impairment of speech (slow, slurred, or aphasia) (5%-21%), and hemiparesthesia (2%-3%). Optic neuritis is reported in 12% to 16% of patients with ADEM.4 Fever and meningeal signs are also frequently reported.5,27 In the present study, the most common symptom reported was change in the level of consciousness (77.8% of patients), followed by pyramidal signs (50%), tonic-clonic seizures (33.4%), visual changes (22.2 %), and urinary disorders (16.7%). Other findings also present in some cases were speech disorders, ataxia, and sensory deficits, in agreement with findings from previous studies.
Imaging exams, especially MRI, are extremely relevant for confirming the diagnosis of ADEM. The main MRI findings were T2/FLAIR hyperintense, bilateral, irregular, and asymmetric lesions. Such demyelinating and inflammatory changes are found, in most cases, in the supra- and infratentorial regions, mainly involving the subcortical and central white matter and cortical gray matter, and may also involve the thalamus and basal ganglia. These lesions generally become hypointense on T1-weighted images of MRI and on CT scan.25,26,28 In addition, in some cases, there may be lesions in the periventricular region, although the classic description of the ADEM lesion pattern on MRI states that this area is preserved.26,27 In this context, based on the cases shown in Table, it appears that all patients (100%) had T2 hyperintense lesions on MRI. Among the most affected regions, the thalamus (27.8%), the spinal cord (22.2%), the brainstem (22.2%), and the cerebellum (22.2%) stand out. The corpus callosum and basal ganglia were also affected areas. In addition, 38.9% of patients developed radiological changes in the periventricular region. This result is similar to that of the study by Koelman et al., in which, of the 228 patients diagnosed with ADEM, 86 (37.7%) had periventricular involvement.25
The prognosis of ADEM is commonly benign, with its evolution influenced by the patient's age, the level of involvement of the CNS, and the time gap between the onset of symptoms and the beginning of treatment. Patients who have the highest risk of neurological sequelae are elderly individuals and those who maintain symptoms after treatment.3 Regarding the evolution of the patient in the post-treatment period, nine patients in the present study had complete neurological recovery and one patient died of complications. The others had sequelae mainly related to urinary incontinence and motor skills.
Conclusion
ADEM is an encephalopathy that can occur after infections caused by Zika, dengue, or chikungunya viruses, common arboviruses in the Brazilian context. For the correct diagnosis of this encephalopathy, it is extremely important to consider previous manifestations of infectious diseases and ADEM’s neurological manifestations; as well as radiological findings, especially those obtained from MRI, with the aim of ensuring adequate follow-up for affected patients, aiming at the best prognosis and minimizing sequelae.