Introduction
Urinary tract infections (UTIs) constitute one of the most common health problems around the world. UTIs affect over 150 million people every year, which entails 10 million ambulatory hospital visits and an estimated cost of 2 billion dollars per year in the United States.1,2Escherichia coli is the predominant uropathogen agent for both community-acquired and nosocomial infections, detected in approximately 80% of UTIs worldwide.3 Furthermore, antimicrobial resistance is a current global concern since infections by resistant pathogens are associated with higher mortality and morbidity.4 Globalization favors the spread of those microorganisms; thus, international approaches are important to tackle the problem. However, low- and middle-income countries, such as Dominican Republic (DR), do not usually have the capacity to implement the necessary measures.
Among the different resistant microorganisms, The World Health Organization (WHO) has classified Extended-spectrum beta-lactamase (ESBL)-producing organisms as a group of bacteria that has a major impact on public health around the world.5 ESBL can hydrolyze and confer resistance to penicillins, cephalosporins, and aztreonam, and are inhibited by clavulanic acid or other beta-lactamase inhibitors.6 Moreover, AmpC-type beta-lactamases confer resistance to the same drugs but are not inhibited by most of the beta-lactamase inhibitors.7 ESBL/AmpC are also usually associated with other classes of antimicrobial resistance, limiting antimicrobial treatment options, and leading to higher management costs.8,9,10 Patients suffering from infection caused by ESBL-producing organisms are more likely to receive incorrect treatment and experience longer hospital stays.11,12
Normally, empirical antibiotic treatment for UTIs is initiated without urine culture and antimicrobial susceptibility testing. However, UTIs are frequently caused by contamination from gut bacteria.13 The levels of colonization with ESBL-producing bacteria in healthy carriers are increasing in the community,14 and the epidemiology of these pathogens varies depending on the geographic region. Thus, due to the increasing antimicrobial resistance worldwide, it is important to determine the regional antibiotic susceptibility through time to update and improve the recommendations for empirical treatment.
Consequently, our research group is doing an effort to expose the underlying antimicrobial resistance among strains isolated from Dominican patients.15,16 The aim of the current study was to assess the prevalence of ESBL and AmpC producing E. coli in urine collected from patients at a tertiary care hospital.
Methods
E. coli strains were collected from urine cultures of outpatients and inpatients at the Hospital Metropolitano de Santiago (HOMS), a tertiary hospital with 400 beds, from November 2019 to February 2020. A total of 72 strains were included in the study.
Inclusion criteria: adult patients at the HOMS with E. coli strains isolated from their urine culture and the informed consent form signed. Exclusion criteria: underage patients; patients who decided to leave the investigation; patients with known diagnosis of a disabling neuropsychiatric disease; non-viable or contaminated bacterial isolates.
The document was created by the authors: Section number one registered general information from the patient (out- or inpatient, gender, age, past medical history, and final diagnosis). Section two enumerated the most frequent risk factors for bacterial resistance, according to the literature (Table 3).17,18 Section three recorded the antibiogram results. Section four listed the results of the screening for ESBL and AmpC producing organisms.
This study received approval by the bioethics committee of Faculty of Health Sciences of the Pontificia Universidad Católica Madre y Maestra (COBE-FACS) (MED-003-1-2018-2019).
Outpatients were contacted in the billing area of the laboratory, where they read and signed the informed consent and provided their contact information. Inpatients who underwent urine cultures were visited in their rooms. After confirmation of the presence of E. coli in the urine sample, each participant was phone called (or visited again in their room) to fill out the data collection instrument.
Specimens were collected and processed following conventional microbiological procedures following the automated systems’ manuals. Pathogen identification and susceptibility tests were carried out with the BD Phoenix (BD Diagnostic Systems, MD, USA) or Microscan® (Beckam Coulter, GA, USA) automated systems at the hospital’s clinical laboratory.
ESBL/AmpC screening
All the E. coli strains collected for the study were screened for ESBL and/or AmpC production. They were cultured in McConkey agar and incubated at 37°C for 12 to 24 hours. Next, a suspension with saline solution and fresh bacteria equivalent to 0.5 McFarland turbidity standards was spread on Mueller Hinton agar (MHA) following the standard procedure for Kirby-Bauer disk diffusion method recommended by the Clinical and Laboratory Standards Institute (CLSI, 2017).19 The screening was carried out with the ESBL+AmpC screen disc kit (Liofilchem Laboratory, Italy). This kit follows the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guideline for the detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance, Version 1.0, 2013. Briefly, 4 disks were applied on the MHA (cefotaxime 30 μg, cefotaxime 30 μg + clavulanic acid, cefotaxime 30 μg + cloxacillin, cefotaxime 30 μg + clavulanic acid + cloxacillin), far enough from each other to allow measuring of inhibition zones. The plates were incubated at 37°C for 18 to 24 hours before the inhibition zones were measured and analyzed following the manufacturer’s interpretation parameters.
Data was cleaned up in Microsoft Office Excel 365 and the descriptive statistics was performed with SPSS ® Statistics software, version 17.0. Chi-square test and univariate analysis were used to determine the statistical significance (p ≤ 0.05) and the Odds Ratio (OR) with 95% confidence interval (CI), respectively.
Results
From November 2019 to February 2020, 184 patients who underwent urine culture were recruited for the study, although half of them had a negative result. Of the 92 positive urine cultures, 72 (78.3%) were reported to have bacterial growth identified as E. coli by the automated systems (BD Phoenix or Microscan). Other common uropathogens detected were Klebsiella pneumoniae (5.4%), Enterobacter cloacae (3.3%), Klebsiella oxytoca (2.2%), Acinetobacter baumannii (1.1%), Enterobacter aerogenes (1.1%), or Proteus mirabilis (1.1%). Among the 72 patients harboring E. coli strains, 83.3 % were female and 16.7 % male (Fig.).
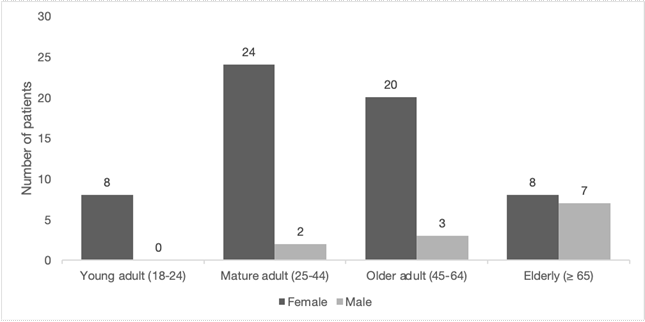
Fig. Classification of patients included in the study by gender and age. Participants of the study were classified by age: young adult (18-24 years old), mature adult (25-44 years old), older adult (45-64 years old), and elderly (≥ 65 years old); and by gender: females are depicted in dark gray, males in light gray.
The 72 isolated strains were screened for ESBL and/or AmpC production. Out of the 72, 31.9% were found to be ESBL producers and 20.8% were classified as AmpC producers. However, 16.7% of those isolates showed both resistant phenotypes simultaneously. Thus, a total of 36.1% showed production of ESBL and/or AmpC. The remaining 63.9% did not show any of the analyzed resistance patterns.
Table 1 illustrates the ESBL/AmpC producing phenotypes detected in the microbiological isolates, distributed by gender and age of the patients from whom the microorganisms were collected. The statistical analysis indicates that female gender, when compared to male gender, is a protector factor for the presence of ESBL/AmpC producing bacteria (p < 0.001; OR 0.07). In the elderly group, 7 patients were men and 8 women. All the isolates collected from men over 65 years old were ESBL/AmpC producers, while among the isolates collected from elderly women, 3 showed no ESBL/AmpC producing phenotype.
Table 1 Classification of E. coli samples isolated from urine cultures based on the production of ESBL/AmpC and the sociodemographic features of the patients from whom the samples were collected
| Sociodemographic features | Total | ESBL/AmpC producing phenotype | Non- ESBL/AmpC producing phenotype |
|
OR (CI 95%)** | |||
|---|---|---|---|---|---|---|---|---|
| Only ESBL | Only AmpC | ESBL + AmpC | Total ESBL/AmpC | |||||
| n = 72 | n = 11 (%) | n = 3 (%) | n = 12 (%) | n = 26 (%) | n = 46 (%) | |||
| Gender | ||||||||
| Female | 60 | 9 (15.0) | 2 (3.3) | 5 (8.3) | 16 (26.7) | 44 (73.3) | < 0.001 | 0.07 (0.01-0.368) |
| Male | 12 | 2 (16.7) | 1 (8.3) | 7 (58.3) | 10 (83.3) | 2 (16.7) | 1.00 (Ref.) | |
| Age | ||||||||
| Under 65 years old | 57 | 7 (12.3) | 2 (3.5) | 5 (8.8) | 14 (24.6) | 43 (75.4) | < 0.001 | 1.00 (Ref.) |
| 65 years or older | 15 | 4 (26.7) | 1 (6.7) | 7 (46.6) | 12 (80.0) | 3 (20.0) | 12.28 (3.03-49.89) | |
Percentage was calculated from the total values of sociodemographic features (rows) and rounded off to one decimal place.
* p-values were calculated by the chi-square test or Fisher’s exact test, when appropriate, comparing the total values of the presence or absence of resistant phenotypes in each variable.
**Odds ratio (OR) was calculated considering ‘female’ and ‘65 years or older’ the risk factors.
Next, the past medical history of the patients was analyzed. The different diseases they reported are listed in Table 2. Hypertension (p = 0.003; OR 4.75) and cancer (p = 0.015; OR 3.09) showed a statistically significant relationship with the detection of ESBL/AmpC producing bacteria in the urine cultures. Additionally, the coexistence of two or more past medical complications is also related to a higher probability of detection of ESBL/AmpC producing bacteria (p = 0.003; OR 4.53), while the lack of underlying medical conditions is related to a lower probability (p = 0.036; OR 0.28). Regarding the most reported risk factors for antimicrobial resistance in urine infections, only urinary catheter placement (p = 0.004; OR 4.77) revealed a statistically significant relationship with the detection of ESBL/AmpC producing bacteria in the enlisted patients.
Table 2 Classification of E. coli samples isolated from urine cultures based on the production of ESBL/AmpC and the clinical characteristics of the patients from whom the samples were collected
| Clinical characteristics | Total | ESBL/AmpC producing phenotype | Non- ESBL/AmpC producing phenotype |
|
OR (CI 95%) | |||
|---|---|---|---|---|---|---|---|---|
| Only ESBL | Only AmpC | ESBL + AmpC | Total ESBL/AmpC | |||||
| n = 72 | n = 11 (%) | n = 3 (%) | n = 12 (%) | n = 26 (%) | n = 46 (%) | |||
| Past Medical History (PMH) | ||||||||
| Recurrent Urinary Tract Infections | 14 | 2 (14.3) | 3 (21.4) | 3 (21.4) | 8 (57.1) | 6 (42.9) | 0.068 | 2.96 (0.89-9.79) |
| Nephrolithiasis | 15 | 3 (20.0) | 1 (6.7) | 2 (13.3) | 6 (40.0) | 9 (60.0) | 0.725 | 1.23 (0.38-3.96) |
| Hypertension | 21 | 5 (23.8) | 1 (4.8) | 7 (33.3) | 13 (61.9) | 8 (38.1) | 0.003 | 4.75 (1.60-14.02) |
| Diabetes | 11 | 3 (27.3) | 0 (0.0) | 3 (27.3) | 6 (54.5) | 5 (45.4) | 0.188 | 2.46 (0.67-9.04) |
| Cancer | 4 | 1 (25.0) | 1 (25.0) | 2 (50.0) | 4 (100.0) | 0 (0.0) | 0.015 | 3.09 (2.19-4.36) |
| Urinary tract disorders | 4 | 0 (0.0) | 0 (0.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 0.616 | 1.83 (0.24-13.85) |
| Nephrological disorders | 5 | 2 (40.0) | 0 (0.0) | 0 (0.0) | 2 (40.0) | 3 (60.0) | 1.000 | 1.19 (0.18-7.65) |
| No PMH | 22 | 1 (4.5) | 0 (0.0) | 3 (13.6) | 4 (18.2) | 18 (81.8) | 0.036 | 0.28 (0.08-0.95) |
| Others** | 22 | 5 (20.8) | 0 (0.0) | 3 (16.7) | 8 (37.5) | 14 (62.5) | ||
| Coexistence of PMH | ||||||||
| Two or more Medical Conditions | 28 | 7 (25.0) | 2 (7.1) | 7 (25.0) | 16 (57.1) | 12 (42.9) | 0.003 | 4.53 (1.62-12.67) |
| Risk factors*** | ||||||||
| Previous Urinary Tract Infection | 38 | 5 (13.2) | 3 (7.9) | 8 (21.1) | 16 (42.1) | 22 (57.9) | 0.263 | 1.74 (0.65-4.64) |
| Previous antibiotic therapy | 41 | 8 (19.5) | 2 (4.9) | 6 (14.6) | 16 (39.0) | 25 (61.0) | 0.554 | 1.34 (0.50-3.58) |
| Urinary catheter | 19 | 4 (21.1) | 1 (5.3) | 7 (36.8) | 12 (63.2) | 7 (36.8) | 0.004 | 4.77 (1.56-14.55) |
| Urological procedures | 5 | 1 (20.0) | 0 (0.0) | 1 (20.0) | 2 (40.0) | 3 (60.0) | 1.000 | 1.19 (0.18-7.65) |
| Coexistence of Risk factors | ||||||||
| Two or more Risk factors | 32 | 6 (18.8) | 2 (6.3) | 7 (21.9) | 15 (46.9) | 17 (53.1) | 0.089 | 2.32 (0.87-6.21) |
Percentage was calculated from the total values of clinical characteristics (rows) and rounded off to one decimal place.
* p-values were calculated by the chi-square test or Fisher’s exact test, when appropriate, comparing the total values of the presence or absence of resistance phenotypes in each variable.
**‘Others’ include PMH of cardiovascular, respiratory, gastrointestinal, neurologic, and rheumatologic diseases.
***Risk factors such as previous UTI and antibiotic therapy were considered in the period of 3 months prior patient approach. However, urinary catheter or urological procedures were considered in the period of 12 months prior to the study.
Thereafter, the antibiotic susceptibility of the microbiological isolates reported by the hospital’s automated system was analyzed (Table 3). As expected, cephalosporins showed low activity against the ESBL+AmpC producing organisms (8.3-16.7% of sensitivity). The bacterial group producing only ESBL showed lower resistance in general, but with a high variability depending on the antibiotic (36.4-81.8% of sensitivity), whilst the non-ESBL/AmpC producing bacteria showed high sensitivity to all of them (84.8% or more).
Table 3 Classification of E. coli samples isolated from urine cultures based on the production of ESBL/AmpC and their antibiotic susceptibility
| Antibiotic susceptibility | ESBL/AmpC producing phenotype | Non- ESBL/AmpC producing phenotype |
|
OR (CI 95%) | ||||
|---|---|---|---|---|---|---|---|---|
| Only ESBL | Only AmpC | ESBL + AmpC | Total ESBL/AmpC | |||||
| n = 11 (%) | n = 3 (%) | n = 12 (%) | n = 26 (%) | n = 46 (%) | ||||
| Aminoglycoside | ||||||||
| Amikacin | Sensitive | 11 (100.0) | 3 (100.0) | 12 (100.0) | 26 (100.0) | 45 (97.8) | 1.000 | 1.57 (1.32-1.88) |
| Resistant | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | |||
| Gentamicin | Sensitive | 7 (63.6) | 3 (100.0) | 4 (33.3) | 14 (53.8) | 38 (82.6) | 0.009 | 4.07 (1.37-12.04) |
| Resistant | 3 (27.3) | 0 (0.0) | 8 (66.7) | 11 (42.4) | 8 (17.4) | |||
| Intermediate | 1 (9.1) | 0 (0.0) | 0 (0.0) | 1 (3.8) | 0 (0.0) | |||
| Carbapenems | ||||||||
| Ertapenem** | Sensitive | 11 (100.0) | 2 (66.7) | 9 (81.8) | 22 (88.0) | 37 (86.0) | 1.000 | 0.84 (0.19-3.70) |
| Resistant | 0 (0.0) | 1 (33.3) | 2 (18.2) | 3 (12.0) | 6 (14.0) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Imipenem | Sensitive | 11 (100.0) | 3 (100.0) | 11 (91.7) | 25 (96.2) | 44 (95.7) | 1.000 | 0.88 (0.07-10.19) |
| Resistant | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (3.8) | 1 (2.2) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | |||
| Meropenem | Sensitive | 11 (100.0) | 2 (66.7) | 10 (83.3) | 23 (88.5) | 44 (95.7) | 0.344 | 2.87 (0.44-18.41) |
| Resistant | 0 (0.0) | 1 (33.3) | 1 (8.3) | 2 (7.7) | 2 (4.3) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (3.8) | 0 (0.0) | |||
| Cephalosporins | ||||||||
| Cefepime | Sensitive | 4 (36.4) | 2 (66.7) | 2 (16.7) | 8 (30.8) | 39 (84.8) | <0.001 | 12.53 (3.94-39.91) |
| Resistant | 3 (27.2) | 1 (33.3) | 10 (83.3) | 14 (53.8) | 5 (10.9) | |||
| Intermediate | 4 (36.4) | 0 (0.0) | 0 (0.0) | 4 (15.4) | 2 (4.3) | |||
| Ceftazidime | Sensitive | 9 (81.8) | 3 (100.0) | 2 (16.7) | 14 (53.8) | 43 (93.5) | <0.001 | 12.28 (3.02-49.89) |
| Resistant | 2 (18.2) | 0 (0.0) | 6 (50.0) | 8 (30.8) | 3 (6.5) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 4 (33.3) | 4 (15.4) | 0 (0.0) | |||
| Ceftriaxone | Sensitive | 4 (36.4) | 2 (66.7) | 1 (8.3) | 7 (26.9) | 39 (84.8) | <0.001 | 15.12 (4.63-49.33) |
| Resistant | 7 (63.6) | 1 (33.3) | 11 (91.7) | 19 (73.1) | 6 (13.0) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | |||
| Cefuroxime | Sensitive | 4 (36.4) | 3 (100.0) | 1 (8.3) | 8 (30.8) | 39 (84.8) | <0.001 | 12.53 (3.94-39.90) |
| Resistant | 7 (63.6) | 0 (0.0) | 11 (91.7) | 18 (69.2) | 6 (13.0) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | |||
| Fluoroquinolones | ||||||||
| Ciprofloxacin | Sensitive | 5 (45.5) | 3 (100.0) | 2 (16.7) | 10 (38.5) | 33 (71.7) | 0.006 | 4.06 (1.46-11.24) |
| Resistant | 6 (54.5) | 0 (0.0) | 10 (83.3) | 16 (61.5) | 13 (28.3) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Levofloxacin | Sensitive | 5 (45.5) | 3 (100.0) | 2 (16.7) | 10 (38.5) | 32 (69.6) | 0.010 | 3.65 (1.33-10.03) |
| Resistant | 6 (54.5) | 0 (0.0) | 10 (83.3) | 16 (61.5) | 13 (28.2) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | |||
| Penicillins | ||||||||
| Amoxicillin/ clavulanic acid | Sensitive | 6 (54.5) | 3 (100.0) | 4 (33.3) | 13 (50.0) | 36 (78.3) | 0.013 | 3.60 (1.27-10.18) |
| Resistant | 1 (9.1) | 0 (0.0) | 2 (16.7) | 3 (11.5) | 3 (6.5) | |||
| Intermediate | 4 (36.4) | 0 (0.0) | 6 (50.0) | 10 (38.5) | 7 (15.2) | |||
| Ampicillin | Sensitive | 2 (18.2) | 1 (33.3) | 1 (8.3) | 4 (15.4) | 14 (30.4) | 0.157 | 2.41 (0.69-8.29) |
| Resistant | 9 (81.8) | 2 (66.7) | 11 (91.7) | 22 (84.6) | 31 (67.4) | |||
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | |||
| Other | ||||||||
| Piperacillin/ tazobactam | Sensitive | 10 (90.9) | 3 (100.0) | 12 (100.0) | 25 (96.2) | 44 (95.7) | 1.000 | 0.88 (0.07-10.19) |
| Resistant | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | |||
| Intermediate | 1 (9.1) | 0 (0.0) | 0 (0.0) | 1 (3.8) | 1 (2.2) | |||
Percentage was calculated from the total values of resistant or non-resistant phenotypes (columns) and rounded off to one decimal place.
* p-values were calculated by chi-square test or Fisher’s exact test, when appropriate, comparing the total values of the presence or absence of resistance phenotypes in each antibiotic. Indeterminate susceptibility was classified as resistant for p-value calculation.
**The number of isolates tested against ertapenem is lower (68 instead of 72) due to the use of two different automated systems in the microbiology laboratory (BD Phoenix and Microscan), as the drugs tested with each system differ.
Amoxicillin/clavulanic acid, one of the most common treatments for urinary tract infection, presented moderately high activity, regardless of the type of strain and the resistance phenotype. Fluoroquinolones stand out for the resistance exhibited by ESBL (54.5%) and ESBL+AmpC (83.3%) producing strains (p = 006; OR 4.06, and p = 0.01; OR 3.65, respectively). Carbapenem antibiotics, on the other hand, were more active, with only the AmpC and AmpC+ESBL producing organisms showing some resistance (8.3-33.3%). Moreover, resistance to carbapenems was also present among the non-producing strains (2.2-14.0%). Amikacin and piperacillin/tazobactam were the most active drugs against the isolates tested, followed by imipenem (Table 3).
Finally, the multidrug resistance (MDR) patterns of the isolated strains were analyzed, considering MDR when resistant to 1 or more drugs in at least 3 different antibiotic categories. A total of 63.9% of the analyzed E. coli strains were classified as MDR. Most of the ESBL/AmpC producing isolates were MDR (84.6%), while the prevalence of MDR reached 52.2% among the non-producing strains.
Discussion
In DR there is little information about the incidence of antimicrobial resistance,15,16 however, these data are essential to prescribe the appropriate empiric therapy to treat common infections.20 This study aims to analyze the antimicrobial resistance and the production of ESBL and AmpC enzymes among E. coli isolates collected from urine, as a first approach to tackle the antimicrobial resistance problem.
The results presented in this study are in accordance to the global pattern, both regarding the frequency of detection of E. coli from positive urine cultures3 and the prevalence of ESBL among the isolates, compared with the reports from other developing countries21,22,23,24 or some locations in the US.25 Furthermore, the prevalence of AmpC is also similar to that reported from other developing countries,26 but much higher than described from developed countries.27
Hypertension was confirmed as a risk factor to harbor E. coli strains that produced at least one of the analyzed resistant phenotypes, supporting previous findings in other countries.28 Consistent with our results, Castillo et al21 also reported an incidence of ESBL producing organisms in 100% of their cancer patients. However, both studies included a very limited number of patients (4 and 6 respectively). A wider study carried out in the USA described only 9.2% of ESBL producing isolates among urine cultures from cancer patients.29 As these patients are immunosuppressed and more frequently exposed to antibiotic therapy, it would be interesting to carry out a specific and wider study to check the incidence of antimicrobial resistance among them in a developing country, which usually report higher frequency of antimicrobial resistance.
On the other hand, suffering recurrent urinary tract infections has been commonly reported as an important risk factor of infection by a resistant microorganism.11,17 This could be caused by an inadequate adherence to the antibiotic treatment, such as stopping the treatment at an early stage, not allowing a full recovery. The results from our study follow that trend, but a largest number of isolates should be analyzed to determine its consistency.
Furthermore, the frequency of ESBL varies among different studies in patients with urinary catheters, from 100% in nosocomial acquired UTI,17 to 47-60% in patients with indwelling urinary catheters,18 confirming our findings. Those high frequencies are to be expected due to the manipulation of the urinary tract and the pathway it may represent for uropathogens to colonize and cause UTI, increasing the need of antibiotic therapy.
Resistance to third generation cephalosporins in E. coli isolates from DR is disturbingly elevated, reaching 32.8% among pediatric patients15 and 51.1% among adults.16 In our present study, resistance to the third generation cephalosporins ceftriaxone and ceftazidime among all E. coli isolates was lower to those found in the previous studies. Ceftazidime is a third-generation cephalosporin not hydrolyzed by ESBL and generally prescribed for UTIs.20 The low sensitivity to ceftazidime reported in this study is not a unique finding among E. coli strains isolated from urine,22,26 but a worrying one, as it limits the use of this antibiotic to treat UTIs.
Previous reports have already described that ESBL producing organisms isolated from urine exhibit considerable co-resistance to many of the currently used antibiotics, hindering the management of UTIs caused by these bacteria.11,22,25 It is important to assess the resistant profiles of the local pathogens to update the antibiotic prescription guidelines and avoid treatment failure. Moreover, previous studies have reported a similarly low resistance to carbapenems.15,16 As carbapenems are the antibiotic of choice for the treatment of ESBL-producing pathogens,28 its use must be carefully limited to avoid loss of efficacy.
Lastly, the elevated frequency of MDR among the analyzed organisms found in this study and other developing countries such as Saudi Arabia,23 Nepal,22 or Ethiopia30 is extremely alarming. Developed countries, however, report a lower incidence of MDR among UTI patients.31 MDR is exacerbated by different factors, including limited therapeutic and diagnostic options, lack of health education among the general population, inadequate drug prescription, under-the-counter antimicrobial sales, and lack of drug regulation.32 Those factors are widespread in developing countries, such as DR, hindering the battle against antimicrobial resistance.
Conclusion
The elevated prevalence of antimicrobial resistance found in one of the hospitals of Santiago de los Caballeros underline the urgency of implementing national and global measures to tackle the problem, especially in developing countries such as the Dominican Republic, where resources are scarce.














