Meu SciELO
Serviços Personalizados
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Cubana de Hematología, Inmunología y Hemoterapia
versão impressa ISSN 0864-0289
Rev Cubana Hematol Inmunol Hemoter vol.30 no.1 Ciudad de la Habana jan.-mar. 2014
ARTICULO ORIGINAL
Moderate dose of hydroxyurea in children with sickle cell disease and stroke. Preliminary results
Dosis moderadas de hidroxiurea en niños con drepanocitosis y accidente vascular encefálico. Resultados preliminares
Dr. Sergio Machín-García, I Dra. Andrea Menéndez-Veitía, I Dr. Claudio Scherle-Matamoros,II Prof. DraC. Eva Svarch,I Dr. Alejandro González-Otero,I Dr. Alberto Arencibia-Núñez,I Dra. Adys Gutiérrez-Díaz,I Dra. Rosa M. Lam-Díaz.I
I Instituto de Hematología e Inmunología, La Habana, Cuba.
II Hospital Clínico Quirúrgico "Hermanos Ameijeiras", La Habana, Cuba.
ABSTRACT
Twenty children with sickle cell anemia, two with SC hemoglobinopathy and one with S/â0 thalassemia were treated, with a previous stroke or abnormal ultrasound transcranial Doppler (TCD) flow velocities more than 170 cm/s. The mean follow-up was of 41 ± 19 months. Hydroxyurea (HU) at a dose of 25 mg/kg/day associated with chronic transfusion therapy, was administrated during one year to patients with stroke. Patients with abnormal TCD received only HU at the same dose. There was a significant decrease of stroke (p <0.02) and TCD flow velocities in the right middle cerebral artery (p <0.003). It was necessary to associate chronic transfusion therapy in three children with high velocities in TCD without stroke, due to the lack of response to the treatment with HU. The combination of HU and transfusions during one year can be useful for stroke therapy and prevention.
Key words: sickle cell disease, stroke, hydroxyurea, chronic transfusion.
RESUMEN
Se trataron 20 niños con anemia drepanocítica, dos ellos con hemoglobinopatía SC y uno con S/â0 talasemia con accidente vascular encefálico o Doppler transcraneal con velocidades del flujo sanguíneo mayor de 170 cm/s. La media de seguimiento fue de 41 ± 19 meses. En los pacientes con accidente vascular encefálico se administraron 25 mg/kg/día de hidroxiurea y se realizó régimen de transfusión crónica por un año. En los niños con Doppler transcraneal patológico se administró la hidroxiurea sola en igual dosis. Hubo una disminución significativa del número de accidentes vasculares encefálicos (p <0.02) y de la velocidad del flujo sanguíneo en la arteria cerebral media derecha (p <0.003). En tres niños con velocidades de flujo muy aumentadas en el Doppler transcraneal sin accidente vascular encefálico fue necesario asociar régimen de hipertransfusión por no respuesta al tratamiento. La asociación de hidroxiurea y transfusiones de glóbulos rojos durante un año pueden ser útiles en el tratamiento y prevención del accidente vascular encefálico.
Palabras clave: sicklemia, accidente vascular encefálico, hidroxiurea, transfusión crónica.
INTRODUCTION
Stroke occurs in 5 -10% of children with sickle cell anemia (SCA) in the first decade of life, and the recurrence, without prophylactic therapy, is about 60%.1-3 Chronic transfusion therapy prevents their recurrence in 40% 4-6 but its long term use is limited by serious side effects such as alloimmunization, infections and iron overload, 7,8 mainly in developing countries where regular blood supplies and chelation therapy are not always available. Furthermore, it is well known that a percentage of patients present secondary stroke after stopping treatment.6,9 Maximum tolerated dose of hydroxyurea (MTD-HU) could be an alternative for the prevention of primary or recurrent stroke in sickle cell disease.10-15 Serial phlebotomies associated to HU reduce the risk of recurrent stroke and iron overload.16-18
Ultrasound transcranial Doppler (TCD) has been shown to be a useful, inexpensive and non invasive method to identify children with high risk of stroke.19
The purpose of this report is to evaluate the use of a fixed dose of HU (25 mg/kg/day) associated with a chronic transfusion regime during one year as a stroke treatment and only HU without transfusions for its prevention.
METHODS
Twenty four patients with sickle cell disease (SCD) followed in our institution from January 1999 to December 2011 who had a stroke defined by any new neurological manifestation that last for more than an hour, or had TCD flow velocities more than 170 cm/s were studied. One patient was excluded due to lack of follow up.
The TCD was always performed by the same observer in a TCD T2 DWL Elektronische. Systeme GmbH, Germany. All patients were evaluated monthly during the first 6 months and every 3 months afterwards. Physical examination and complete blood counts were done at each visit. Hemoglobin and reticulocytes count from patients on chronic transfusion were not included until three months after stopping transfusions; serum alanino-aminotransferase (ALT), creatinine and fetal hemoglobin (HbF) were determined every 3 months. HbF was not determined until stopping transfusions. All techniques were carried out by methods standardized in our institution.
HU (DUREA, Puerto Rico Pharmaceutical, Inc.) at a fixed dose of 25 mg/kg/day associated with chronic red cell transfusions (10 mL/kg) was administered in patients with stroke during one year. Children with abnormal TCD were only treated with HU at the same dose.
Treatment was interrupted if any of the following events occurred: hemoglobin declined more than 2 g/dL from the baseline level, WBC < 3 x 109/L and platelets < 80 x 109/L.
The study was approved by the Institutional Scientific and Ethic Committees. Parents' informed consent was obtained.
Wilcoxon rank-sum test was used for clinical events and the paired Student t-test for hematological parameters; P values less than 0.05 were considered statistically significant.
RESULTS
Twenty three patients were included in the study (20 SCA, two SC hemoglobinopathy and one S/â0 thalassemia); this group represented 11.5% of total patients. Thirteen were boys. The mean of age was 10 ± 4.3 years and mean follow-up time was of 41 ± 19 months.
Eleven patients were treated for an increase TCD flow velocities, 11 by stroke and one with stroke and accelerate TCD flow velocities.
Stroke decreased significantly with the treatment (p <0.02).
There was a significant increase in total hemoglobin after treatment (table 1). There were no significant changes in ALT, creatinine, reticulocytes, WBC and platelets values. Although there was an increase of HbF (3.1% to 6.7% after treatment) it was not statistically significant.
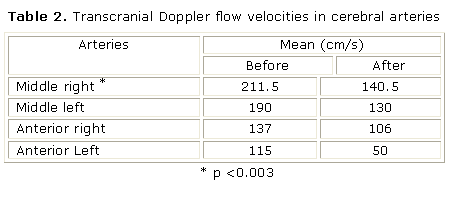
A significant decrease of TCD flow velocities in middle right cerebral artery was observed (p <0.003) (table 2), but it was necessary to associate a chronic transfusion regime in three children because they did not respond to only HU treatment alone.
No secondary effects were observed and it was not necessary to interrupt treatment in any case.
DISCUSSION
In our series stroke decreased significantly, similar to patients treated else where with MTD-HU.10-18 There was an increase of HbF as previously described,20-22 but it was not statistically significant; this could be related to the impossibility of performing initial determination in 12 patients with stroke, because an emergency exchange transfusion was done at admission. Significant increase of hemoglobin was observed the same as it has occurred in other studies.21 Reticulocyte, WBC and platelets did not change, but clinical benefits were achieved. This observation could be explained by other mechanisms of HU actions in SCD.
Although lower TCD flow velocities were observed in all arteries after therapy, they were only significant in the middle right cerebral artery, which may be explained by the small number of patients included.
There are many studies about HU and SCD. In the United States, MTD-HU are usually used but some studies have found significant improvement of the patient´s condition with a fixed dose of 15 - 25 mg/kg/day. 22-24 Although preliminary, the results of this study suggest that moderate dose of HU associated to a short period of chronic transfusions can decrease stroke frequency and prevent its recurrence in children with SCD. On the other hand, there are no secondary effects, treatment is economically less expensive and allows treating a higher number of patients.
Our results support that in SCD, HU is useful in stroke treatment and in its prevention when chronic transfusions for long periods are not possible.
REFERENCES
1. Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood 1998 Jan;91(1):288-94. PMID:9414296.
2. Powars D, Wilson B, Imbus C, Pegelow C, Allen J. The natural history of stroke in sickle cell disease. Am J Med 1978;65(3):461-71. PMID: 717414.
3. Ballas SK, Kesen MG, Goldberg MF, Lutty GA, Dampier C, Osunkwo I, et al. Beyond the Definitions of the Phenotypic Complications of Sickle Cell Disease: An Update on Management. Scientific World J. 2012; 2012: 949535. Published online 2012 August 1. PMCID: 3415156.
4. Sarnaik S, Soorya D, Kim J, Ravindranath Y, Lusher J. Periodic transfusions for sickle cell anemia and CNS infarction. Am J Dis Child 1979 Dec;133(12):1254-7. PMID: 517476.
5. Sarnaik S, Lusher J. Neurological complications of sickle cell anemia. Am J Pediatr Hematol Oncol 1982 Winter;4(4):386-94. PMID: 7168488.
6. Scothorn D, Price C, Schwartz D, Terrill C, Buchanan GR, Shurney W, et al. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr 2002 Mar;140(3):348-54. PMID: 11953734.
7. Castellino SM, Combs MR, Zimmerman SA, Issitt PD, Ware RE. Erythrocyte autoantibodies in paediatric patients with sickle cell disease receiving transfusion therapy: Frequency, characteristics and significance. Br J Haematol 1999 Jan;104(1):189-94. PMID: 10027733.
8. Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T, et al. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood 2000 Jul;96(1):76-9. PMID: 10891433.
9. Adams RJ, Brambilla D,STOP2-Trial-Investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med 2005 Dec;353(26):2769-78. PMID:16382063.
10. Ali SB, Moosang M, King L, Knight-Madden J, Reid M. Stroke recurrence in children with sickle cell disease treated with hydroxyurea following first clinical stroke. Am J Hematol 2011 Oct;86(10):846-50. PMID: 21898530.
11. Ware R, Aygun R. Advances in the use of Hydroxyurea. Hematology Am Soc Hematol Educ Program 2009;2009:62-69.
12. DeBaun MR. Secondary prevention of overt strokes in sickle cell disease: therapeutic strategies and efficacy. Hematology Am Soc Hematol Educ Program 2011;2011:427-33.
13. Zimmerman SA, Schultz WH, Burgett S, Mortier NA, Ware RE. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood 2007 Aug 1;110(3):1043-7. PMID: 17429008.
14. Aygun B, McMurray MA, Schultz WH, Kwiatkowski JL, Hilliard L, Alvarez O et al Chronic transfusion practice for children with sickle cell anaemia and stroke. Br J Haematol 2009 May;145(4):524-8. PMID: 19344396.
15. Bernaudin F, Verlhac S, Arnaud C, Kamdem A, Chevret S, Hau I, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood 2011 Jan 27;117(4):1130-40. PMID: 21068435.
16. Ware RE, Helms RW; SWiTCH Investigators. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH). Blood 2012 Apr 26;119(17):3925-32.
17. Greenway A, Ware RE, Thornburg CD. Long-term results using hydroxyurea/phlebotomy for reducing secondary stroke risk in children with sickle cell anemia and iron overload. Am J Hematol 2011 Apr;86(4):357-61. PMID: 21442640.
18. Ware RE, Schultz WH, Yovetich N, Mortier NA, Alvarez O, Hilliard L, et al. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH): A Phase III Randomized Clinical Trial for Treatment of Children With Sickle Cell Anemia, Stroke, and Iron Overload. Pediatr Blood Cancer 2011 Dec 1;57(6):1011-7. PMID: 21826782.
19. Bulas D. Screening children for sickle cell vasculopathy: guidelines for transcranial Doppler evaluation. Pediatr Radiol 2005 Mar;35(3):235-41. PMID: 15703903.
20. Steinberg MH, Lu ZH, Borton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: Determinants of response to hydroxyurea. Multicenter Study of Hydroxyurea. Blood 2001 Feb 1;89(3):1078-88. PMID: 9028341.
21. Zimmerman SA, Shultz NH, Davis JS, Pickens CV, Mortier NA, Howard TA et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood 2004 Mar 15;103(6):2039-45. PMID: 14630791.
22. Svarch E, Machín S, Nieves RM, Mancia de Reyes AG, Navarrete M, Rodríguez H. Hydroxyurea treatment in children with sickle cell anemia in Central America and the Caribbean Countries. Pediatr Blood Cancer 2006 Jul;47(1):111-2. PMID: 16550531.
23. Ferster A, Tariri P, Vermylen C, Sturbois G, Corazza F, Fondu P, et al. Five years of experience with hydroxiurea in children and young adults with sickle cell disease. Blood 2001 Jun 1;97(11):3628-32. PMID: 11369660.
24. Braga LB, Ferreira AC, Guimaraes M, Nazario C, Pacheco P, Miranda A et al. Clinical and laboratory effects of hydroxiurea in children and adolescents with sickle cell disease: a Portuguese hospital study. Hemoglobin 2005;29(3):171-80. PMID: 16114180.
Recibido: Mayo 9, 2013
Aceptado: Junio 21, 2013
Dr. Sergio A Machín García. Instituto de Hematología e Inmunología. Apartado 8070, La Habana, CP 10800, CUBA. Tel (537) 643 8695, 8268. Fax (537) 644 2334. Correo electronico: rchematologia@infomed.sld.cu