INTRODUCTION
Complement system includes three pathways: classical, alternative and lectin pathway.
The last one is present since from the invertebrates, but it is not yet well defined. It is still discovering new structures and new evidences of its component’s functions.
So far there are five different forms of pattern recognition (PRM): mannose binding lectin (MBL); H-, L- and M-ficolin (also known as Ficolin-3, -2 and -1, respectively); and collectin K (CL-K1): an MBL (MASP) -1 and -2 are associated with the serine protease to activate the complement. In addition, CL-K1 and collectin L 1 (CL-L1) are related to heteromers that are also associated with MASPs and activate complement. 1
These initiators are not capable of initiating the enzymatic cascade by themselves; it needs other structures with the enzymatic characteristics that become the beginning of this activity, such as MASP2.
Although MASP-2 is capable of self-activation, recent studies have shown that MASP-1 is the exclusive activator of MASP-2 under physiological conditions. 1,2 MASP3 and the MAp44 are components with regulatory functions in this pathway.
These proteins are found in the highest percentage in the blood and they diffuse through the blood-brain barrier, but they can also be synthesized in the central nervous system.
These proteins participate in enzymatic reactions in the lectin pathway, where a balance is established between the activated and non-activated forms and between the soluble forms and those associated with other surfaces.
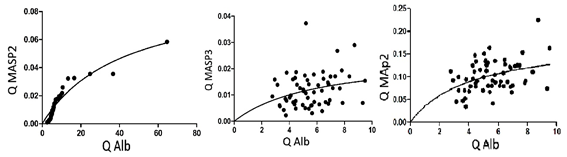
When the variation of Q albumin as a measure of protein diffusion between blood and CSF is plotted against the quotient diffusion of some of the lectin pathway, a typical curved curve is observed that resembles the enzymatic reactions shape, such as Michaelis-Menten.reaction.
The objective of this work is to evaluate the behavior of the lectin pathway component MASP2, MASP3 and MAp44, based on their observed dynamics mimicking enzymatic activity.
METHODS
MASP2 was quantified in 56 serum and CSF samples by the enzyme-linked immunosorbent assay (ELISA) kits (Hycult Biotech, Uden, the Netherlands). (3)
Plasma levels of MASP3 and Map44 were measured using time-resolved immunofluorometric in duplicates. All assays and specific antibodies for the assays were produced in-house. Detailed description for each assay can be found in the reference. 4
Albumin in serum and in CSF was quantified by nephelometric assay using Behring Nephelometer-Analyzer from Siemens (Marburg, Germany).
Statistical package MedCalc version 13.3.3.0 and GraphPad Prism version 5.01 was used to perform statistics and graphs.
RESULTS
The passage of proteins from the blood to the CSF is characterized from the values of Q Albumin. The distribution of the QMASP2, QMASP3 and QMap44 are shown in Fig. 1.
In the following table 1 you can see the new constants Qmax and Kdacw calculated from the similarity with the distribution of the Michaelis-Menten reaction.
DISCUSSION
The passage of these components of lectin pathway from blood to CSF has allowed characterize these proteins as blood derived with the possibility of being synthesized in the CNS (1) because they follow the established statements for these types of proteins.
MASP2 has a behavior when it is distributed based on the speed of diffusion between the blood and the CSF that resembles a reaction of the Michaelis-menten type. This favors that this curve can be used to characterize this protein whose tracing resembles a reaction of enzymatic activity. The same thing happens with the regulation complement molecules MASP3 and MAp44.5,6)
By this way it was possible to characterize the new constants Kdacw and Qmax for each ones of these components of the lectin pathway.
The Qmax is the maximum value experimentally obtained from the diffusion of the protein when it passes from blood to CSF. It is the maximum relationship between these proteins when they pass from blood to CSF as a function of its serum concentration. It is the maximum diffusion speed between blood and CSF
The Kdacw is the Q value of the evaluated protein from the passage through the blood-LCR barrier that corresponds to the half-maximal speed-It can be experimentally obtained from Qmax / 2 and is a constant value depends on the molecule which is being evaluated.
With these new constants, the passage of these proteins from the serum to the CSF can be better defined and allows improving theoretical and practical analysis of this dynamics in blood-CSF barrier.