Introduction
Stroke represents the third cause of death worldwide, the first of disability, the second of dementia and the most common neurological disorder. The World Health Organization defines stroke as an acute focal neurological deficit lasting more than 24 hours, which may or may not lead to death and whose cause is apparently vascular. Cerebrovascular disease (CVD) also includes a permanent or transient type of cerebral affection that appears after ischemia or bleeding due to damage of the cerebral blood vessels.1
Age is a non-modifiable risk factor of CVD since it provokes modification of the incidence of stroke, particularly of ischemic stroke. Surprisingly, the overall stroke burden as reported in 2010 was greater in individuals younger than 75 years (10 469 624 cases) than in those who are older (6 424 911 cases); being this situation more evident in low and middle-income countries.2 In developing countries, stroke incidence in young and middle-aged adults seems to be increasing, probably attributed to the increase in obesity, hypertension and diabetes mellitus.2 The probability of long-term survival for younger patients is lower than expected,3,4 perhaps due to a high percentage of strokes of undetermined origin (35 %- 4 2%) that are often not correctly diagnosed.5 In addition to the high mortality of stroke, this disease has a great impact due to its morbidity, stroke recurrence and cardiovascular events. It has been considered a devastating disease because of the implications for daily life resulting in disability for work and changes in family and social life. Although it has been reported that younger patients can recover their independence, they will likely be more unemployed than healthier subjects.6
Common risk factors for stroke (atherosclerosis, diabetes, hypertension, familial history of stroke) and uncommon ones (vasoconstriction, migraine, non-atherosclerotic arteriopathies) in elderly and young individuals respectively,6 are likely linked to mechanisms related with the release of many substances (lipoproteins, enzymes, specific nervous system proteins, glycoproteins, inflammatory cytokines, acute phase proteins, adhesion molecules, and others) into the bloodstream from damaged tissue.7 Some of these substances have been used as potential biomarkers for diagnosis and prognosis of stroke patients.8 Nevertheless, neurological scales have provided more efficient outcome prognosis after stroke than these biochemical markers, due to the fact that single blood biomarkers show a moderate sensitivity and specificity. For this reason, biomarker panels have been proposed for a more adequate evaluation.7
In recent years, the assay of some blood markers such as low density lipoprotein-cholesterol and hemoglobin A1c1 have been considered for stroke prevention.7 At the same time, other reports have suggested nervous system specific proteins including N-terminal brain natriuretic peptide, glial fibrillary acidic protein, neuronal specific enolase, calcium dependent fibrillary protein, myelin basic protein, antibodies against the NR2 peptide of the n-methyl D-aspartate receptor. Moreover, the American College of Cardiology and American Heart Association have recommended assaying HgA1c1, C-reactive protein (CRP), lipoprotein-associated phospholipase A2 and microalbuminura (μalbuminuria) to evaluate cardiovascular disease risk.7,8
Despite the expected relationship between the levels of these biomarkers and the occurrence and or prognosis of stroke, most studies have not been conclusive.8,9 The heterogeneity of stroke (etiology, localization and size of infarction, variety of substances released) might be related, but the methods employed and age dependence could also be involved.
In the present study, we evaluated the possible relationship of blood markers with age and clinical characteristics of ischemic stroke patients.
Methods
A cross-sectional study of cases and controls was carried out at the Institute of Neurology and Neurosurgery (INN), Havana, Cuba. Patient universe consisted of all individuals over 18 years of age with an acute focal neurological deficit who attended the Emergency department of the "Comandante Manuel Fajardo" Teaching Clinical Surgical Hospital. Those patients with confirmed diagnosis of acute ischemic stroke by computerized axial tomography (CAT) were included in the first 72 hours of the event, and seven days later CAT scans were repeated to confirm diagnosis. Patients with hemorrhagic stroke or intracerebral hemorrhage due to metastases, brain tumor or aneurysm were excluded. Patients were distributed according to age in two groups: patients ≤ 55 years and > 55. Many studies have considered young stroke subjects when it occurs between the ages of 18 and 50 years. Nevertheless, currently a consensus for this does not exist, and cut-off points for young stroke patients has been considered even up to 55 or 60 years of age.4 In our study, we chose 55 years as a cut-off age. Patients were matched by age and sex with the control subjects. Control subjects were recruited from a health area of the Policlinic “19 de abril”. They consisted of individuals with no personal history of neurological disease, malignancies, chronic inflammatory, collagen disease or other serious systemic diseases; but other comorbidities could be present (arterial hypertension, diabetes mellitus, dyslipidemia). The control subjects were submitted to a physical-neurological examination by a physician specialized in Neurology. Neurological deficit was measured in the stroke group on admission by trained physicians employing the National Institutes of Health Stroke Scale (NIHSS).10
All individuals who participated in the study signed an informed consent that included the reasons for the research and its purposes. In patients who were unable to give their informed consent, relatives or caregivers were asked for authorization. The ethical principles for medical research in human beings of the Declaration of Helsinki of the World Medical Association in 201311 were taken into account for the development of informed consent and submitted for approval to the Ethics Committee of INN.
Ten mL of fasting venous blood were extracted for the hemochemical studies. All blood samples were distributed in dry tubes which were centrifuged at 2000 rpm for 10 minutes to obtain serum. Blood chemistry analysis included: total cholesterol (Chol), triglycerides (TG), glutamic-pyruvic transaminase (GPT), glutamic-oxalacetic transaminase (GOT), gamma glutamyltranspeptidase (GGT), glucose, creatinine and uric acid. The acute phase reactivity proteins measured were: CRP, alpha 1 antitrypsin (AAT), complements 3 and 4 (C3 and C4, respectively), and ceruloplasmin.
Four mL of urine from the first morning urination were collected to evaluate endothelial dysfunction (microalbuminuria). The urine sample was centrifuged at 1000 rpm for 10 min. Serum and urine samples were frozen at -20oC for no more than 30 days until processing.
Biochemical procedures
Reagent kits from Biological Products Company “Carlos J. Finlay”, (Havana, Cuba) were employed for blood chemistry analysis (Chol, TG, GPT, GOT, GGT, glucose, creatinine and uric acid).
Kits from CPM Scientifica (Italy), based on the principle of immunoturbidimetry were used to measure acute phase reactivity proteins (CRP, AAT, C3, C4 and ceruloplasmin).
μalbuminuria was determined by a sandwich immunoenzymatic quantitative assay (UMELISA, Immunoassay Center, Havana, Cuba) employing albumin specific monoclonal antibody coated plates. The fluorescence intensity emitted was read in an ultramicroELISA “SUMA” equipment (Immunoassay Center, Havana, Cuba). International reference values (<20 mg/mL) were considered as reference of normality.
Statistical analysis
Frequencies of clinical and demographic variables were calculated. Normality was tested for continuous variables by the Kolmogorov-Smirnov test. The medians and 10-90 percentiles for the NIHSS and laboratory variables were calculated, and the differences between groups were determined using Mann-Whitney U test or t-student test. Correlations between continuous variables were assessed by Spearman`s correlation coefficient. Associations between categorical variables were demonstrated with the (2 test. Factorial ANOVA was employed to evaluate the association of age and neurological severity of stroke (NIHSS) with serum CRP concentration. The results were processed employing the Statistica 10.0 program for Windows, considering p < 0.05 as the significance level.
Results
Description of demographic and neurological variables
Table 1 shows age and sex of the individuals studied. There were no significant differences between the age of patient and control groups. No differences were observed in gender distribution between patients and controls for the corresponding age ranges.
Table 1 Description of demographic variables and neurological feature for patients and control groups according to age
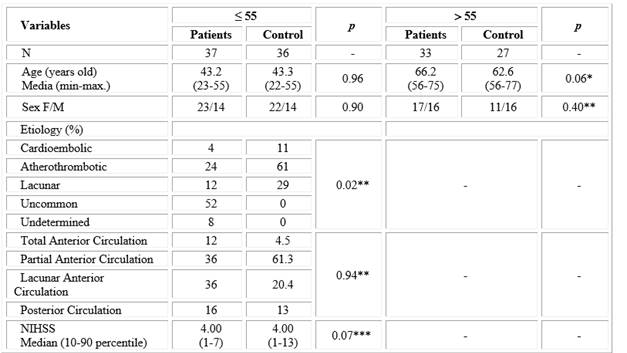
*t-student test. ** X2 test. ***Mann-Whitney test.
Analysis of stroke etiology showed that in the group under 55 years of age, unusual origin predominated (52 %), with a lower frequency of indeterminate etiology (8 %). Contrastingly, the main etiology in the group older than 55 years was atherothrombotic (61 %) and there were no cases of unusual and indeterminate etiology. Considering localization, partial and lacunar strokes of the anterior circulation were equally prevalent (36 %) in patients under 55 years of age, while for those older than 55 years, partial stroke of the anterior circulation was the most frequent (57 %), followed by lacunar stroke (21 %). Neurological impairment, as measured by the NIHSS scale, was similar in both patient groups (Table 1).
Blood chemistry biomarkers, acute phase reactivity proteins and endothelial dysfunction in patients with ischemic stroke and control groups
Significant differences between patients and controls were observed for several components when the results of routine blood chemistry, acute phase proteins and endothelial dysfunction were compared in both age groups (Table 2). Transaminases and GGT were found to be significantly higher in patients with respect to controls in both age groups, except for GPT in patients > 55 years, although mean enzyme activities were within the normal range. Glycemia was higher in patients of both age groups with respect to their control groups. Glucose mean values were above the reference range, but statistical significance was obtained only in the older group.
For acute phase proteins, the concentration of AAT was significantly higher in both groups of patients with respect to their controls, while C3 and ceruloplasmin were significantly increased only in patients ≤ 55 years. μalbuminuria displayed a marked statistical difference in patients (≤ 55 years: 10.59 and > 55 years: 13.35 mg / L) with respect to control subjects (≤ 55 years: 0.02 and > 55 years: 0.00 mg/L), although the mean concentration was within the normal range (Table 2).
Table 2 Comparison of blood biomarkers between ischemic stroke patients and control groups according to age
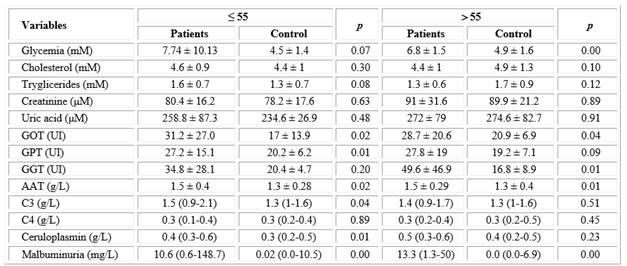
Abbreviations: GOT, glutamic-oxalacetic transaminase; GPT, glutamic-pyruvic transaminase; GGT, gamma glutamyltranspeptidase; AAT, alpha 1 antitrypsin; C3 and C4, complement C3 and C4.
* Data of blood chemistry and AAT are shown as mean± standard deviation: * t- student test. p < 0.05
**Data of C3, C4, ceruloplasmin and μalbuminuria as median (10-90 percentil): Mann-Whitney test. p < 0.05
Association of CRP with the age of ischemic stroke patients
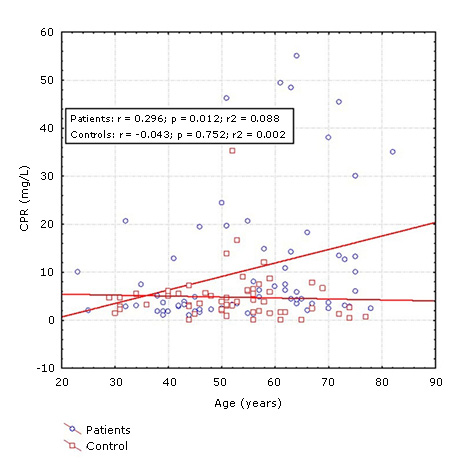
Despite the fact that no differences were observed between the two groups of patients in most of the variables previously studied, CRP had a different behavior. The concentration of this protein was significantly higher in patients over 55 years of age (median: 7.00) compared to those ≤ 55 years (median: 3.20) and with respect to the two control groups (median: ≤ 55 years: 3.80 and > 55 years: 2.10). In ≤ 55 years of age patients did not show statistical differences in relation to the two control groups (Fig. 1). Post hoc power analysis for PCR levels in patients and controls according to age revealed an acceptable statistical power (0.85).
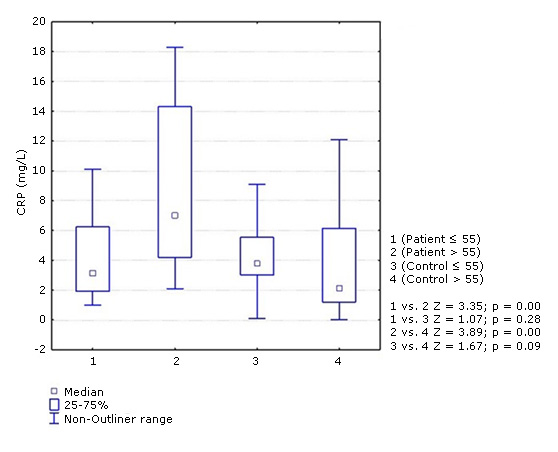
Fig. 1- Concentration of C-reactive protein (CRP) in ischemic stroke patients and controls according to age groups (≤. 55 and > 55 years). Mann-Whitney test. p < 0.05.
Spearman´s correlation test showed a significant correlation between age and CRP concentrations in patients with ischemic stroke, (R = 0.34, p = 0.00) (Fig. 2), however, no significant lineal correlation with age was found in the control group.
Association of CRP with neurological deficit of patients with ischemic stroke.
Factorial ANOVA to evaluate the association of age and neurological severity of stroke (NIHSS) with serum CRP concentration revealed that CRP was significantly associated with the age of ischemic stroke patients, but not with neurological severity. CRP levels were significantly higher in older patients independently of the NIHSS score, although a tendency toward higher levels for older patients was observed in those with more severe neurological impairment (Fig. 3).
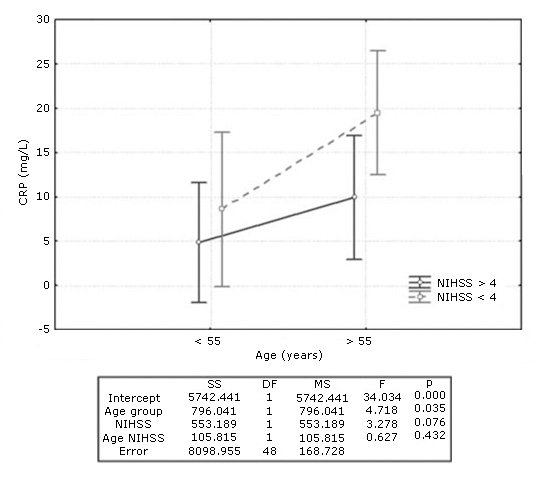
Fig. 3- C-reactive protein (CRP) levels in patients with ischemic stroke according to age and neurological severity. Results of Factorial ANOVA below the graph.
Association of CRP with the etiology of ischemic stroke.
CRP concentration was compared between both groups of patients in association with the etiology of ischemic stroke. Kruskall-Wallis analysis could only be carried out considering aterotrombotic, cardioembolic and lacunar etiologies because uncommon and undetermined etiologies were only seen in patients ≤55 years of age. No significant differences were observed for CRP according to stroke etiology (≤ 55 years: H = 2.09, p = 0.35; > 55 years: H = 2.5, p = 0.28).
Discussion
Several studies seeking to explore the possible usefulness of hemochemical biomarkers in the diagnosis, severity and prognosis of stroke have been reported, but there is no consensus concerning their usefulness in clinical practice,7,8,9 and only a few investigations have focused on the comparison of blood biomarkers among different age groups.12,13 The age of stroke patients could be influencing these results. Differences in stroke etiology related to age have been reported, which might lead to changes in the molecular mechanisms and thus to the substances released into the bloodstream.6,12,13,14 In this sense, we found that among the serum biomarkers studied, only CRP differed between ≤ 55 years and > 55 years of age patients with ischemic stroke, although other acute phase proteins, such as AAT, ceruloplasmin, C3 and μalbuminuria showed higher concentrations in both groups of stroke patients.
Concerning stroke etiology, a predominance of unusual cases of stroke (52 %) was observed in ≤ 55 years stroke patients, followed by an unexpectedly high atherothrombotic contribution (24 %) and lacunar etiology (12 %). A similar study in Barcelona, Spain described that the three most frequent causes were undetermined (30.4 %), lacunar origin (23.9 %) and unusual (16.3 %). However, our results were similar to those reported by Kefi et al. in 2016 who found that ischemic stroke of unusual cause was the most frequent 36 %.15 This variability in the etiologies of ≤55 years adults who have suffered stroke has been informed previously.16,17 The high percentage of unusual ischemic stroke is due to the fact that atherogenic or cardioembolic processes are not common in this age group. Some rare etiologies which are mainly due to blood disorders (antiphospholipid syndrome, protein S deficiency, resistance to Protein C, among others) are more frequent.14
The presence of atherothrombotic stroke in ≤55 years of age patients could be due to changes in the vasculature resulting from an atherogenic profile in our subjects, as a consequence of hypercaloric and hyperlipidemic diets related to unhealthy lifestyles and diets. For our patient group older than 55 years, atherothrombotic etiology (61 %) was the most prevalent and lacunar strokes followed (29 %). In general, our results are in line with what most authors report, but undoubtedly there are great variations in the proportion of patients assigned in the different etiological subgroups,3,15,16,18 mainly related to the operational criteria employed for classification and/or the completeness of the ancillary investigations.17
Concerning the distribution of stroke topography, anterior circulation infarction (partial and lacunar) predominated in both age groups, although partial infarcts prevailed in older patients and lacunar infarcts in the youngest group. Similar results have been reported in other studies.19,20
Neurological deficit as measured by the NIHSS score in both patient groups did not differ statistically; although the percentile range tended to be higher for patients over 55 years of age (13 points). Kawle et al. in 2015 reported similar findings, but in this case our study groups displayed a score corresponding to mild rather than moderate (7-15 points) neurological deficit.12 This could be related with the high percentage of partial and lacunar strokes in both age groups.
Glycemia plays an important role in the mechanism of ischemic stroke. The higher glucose levels observed in the two patient groups, with mean values over the reference range, may possibly be related to the severity of stroke, as depicted in a series of 811 patients, where stroke hyperglycemia was reported to be associated with a higher mortality, even after adjusting the results for other risk factors.21
Other parameters, such as transaminases and GGT, also seem to have a role in the mechanisms of ischemia. We observed an increase in these enzyme activities compared to the control subjects in this study. A similar pattern during the first 7 days after acute stroke was reported by Muscari et al.21 These authors suggest that O-linked (-N-acetylglucosamine transferase is an enzyme related to the metabolism of glutamate through its ability to neutralize glutamate toxicity during the process of excitotoxicity in an ischemic event, and that it seems to be influenced by inflammation21. Furthermore, in a very large Korean population (456 100 participants) increasing GGT was reported to be independently correlated with an increased risk of stroke.22
Among the events participating in ischemic stroke the release of acute phase proteins involved in inflammatory processes are included.23 AAT is an inhibitor of serum proteases with anti-inflammatory, anti-apoptotic and cytoprotective properties during cardiovascular and cerebrovascular events.23 In our study, the higher concentration of AAT in the two patient groups with respect to control individuals is in correspondence with a previous investigation where increased AAT levels were found to be associated with poor prognosis in ischemic stroke patients.24 Nevertheless, recent trends concerning this topic indicate that AAT gene deficiency seems to be associated with the risk of stroke.23 Meanwhile, ceruloplasmin, a protein participating in Cu+2 transport through the bloodstream and also an acute phase and antioxidant protein, was significantly higher with respect to the control group only in patients under the age of 55. In the same way as AAT, increased levels of this protein have also been observed in ischemic stroke.25 Its role in vascular diseases is still uncertain, since it is not known whether this is due to its oxidative function on low-density lipoproteins or its role as an inflammatory marker.26
μalbuminuria, well-known as a marker of endothelial dysfunction,8 was significantly elevated in both groups of patients regardless of their age compared to the control subjects. It have found high levels of μalbuminuria during the acute event of this disease.27 Moreover, Chowdhury et al. in 2012 showed an association between positive μalbuminuria and the age of stroke patients. In this sense, these researchers reported that 13.3 % of patients between 50 and 59 years of age compared to 80 % of those over 60 years had positive μalbuminuria.26
C-reactive protein plays an essential role in the immune response and might be a useful marker of the severity and short-term prognosis of stroke. This protein has been associated with endothelial dysfunction and progression of atherosclerosis, perhaps through the decrease of nitric oxide (NO) synthesis. It has also been linked to the risk of cerebrovascular and cardiovascular events.27 In the guidelines from the centers for control and prevention and the American Heart Association, the concentrations of high-sensitivity CRP have been classified in relation to the possibility of suffering a cardiovascular event, being the values < 1, 1-3 and > 3 mg/L for low, moderate and high cardiovascular risk respectively.28
Our results confirmed that there is an association between CRP and stroke, but only in older stroke patients where CRP concentrations were higher, while patients ≤ 55 years of age displayed no change. In a study of 60 stroke patients aged 51 to 70 years, Konin et al. reported that 54 individuals had CRP levels above the reference value (6 mg/L).27 It has also been shown that the increase of this protein takes place between 12 hours and 72 hours after the acute event of stroke,3,29 corresponding with the timing of our study.
Increased CRP levels in stroke patients over 55 years of age could be related to an association of this protein with the atherogenic and atherothrombotic process. This was supported by the direct correlation that we found between CRP and age of patients and could be related to the ability of CRP to predict vascular damage in favor of a proinflammatory and proatherosclerotic phenotype, through inhibition of NO synthase transcription, facilitating apoptosis and blocking angiogenesis. It also has a significant proatherogenic function, seemingly given by a direct action over activation of type 1 angiotensin receptor synthesis in smooth muscle cells, destabilization of fibrous atheroma plaques through the stimulation of matrix metalloproteinases 1 and the activation of coagulation.29) Some studies that have determined the levels of CRP taking into account the age of patients have found significant differences.30,31 For example, Liu et al. in 2014 stratified a group of ischemic stroke patients according to the values of high-sensitivity CRP, reporting a significant increase with the age of these individuals (43 - 63 years).30 Concomitant with our results, this correlation has not been observed previously in healthy subjects.31 Despite changes in CRP concentrations, these may not necessarily be associated only with age, since genetics, lifestyles and dietary habits of the individuals could also be involved. Another aspect differentiating both age subgroups was the etiology of stroke, which may be a confounding factor, as inflammatory mechanisms may be more relevant in patients with large and small vessel disease (more prevalent in older patients), than in the unusual etiology subgroup, which predominated in ≤ 55 years of age stroke patients. Although in our study CRP concentration did not significantly differ between the different etiologies of ischemic stroke when both age groups were compared, these results are limited because uncommon and undetermined etiologies were only seen in patients ≤ 55 years of age. Nevertheless, whatever the cause may be, the fact is that in ≤ 55 years of age stroke patients elevated CRP is not a distinctive issue, and thus CRP studies may not render the results expected concerning prognosis and severity.
Although in our study CRP values correlated with the age of patients, but not with the NIHSS score, joint analysis of both variables (age and NIHSS) revealed a tendency towards even higher CRP values in older patients with more severe neurological impairment. Mazaheri et al. in 2018 also found association between CRP (( 7 mg/L) and NIHSS score (( 13).32 Another study showed a high correlation between these two parameters in patients with ischemic stroke.13 The association between the elevation of CRP and the severity of stroke is not well understood. However, as atherothrombosis was the most frequent etiology among patients older than 55 years and it is considered an inflammatory disorder, it is possible that acute phase proteins, such as CRP increase in the first hours of the event. CRP elevation may be a direct expression of the extent of brain tissue damage and the severity of stroke that might also contribute to ischemic damage.13
Increased CRP could be a response to the occurrence of acute stroke, but CRP could also be an acceptable indicator of underlying inflammatory mechanisms leading to the acute event due to accumulation of atherogenic, prothrombotic and endothelial dysfunction processes, which are associated with the severity of stroke and aging, mainly in patients older than 55 years. It would be necessary to confirm these results in the future with high-sensitivity CRP. Our findings support the importance of considering age when evaluating the usefulness not only of CRP, but possibly of other blood biomarkers as clinical tools for predicting long or short-term neurological outcome or stroke recurrence events in ischemic stroke patients.