Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Nucleus
versión impresa ISSN 0864-084X
Nucleus n.41 Ciudad de La Habana ene.-jun. 2007
CIENCIAS NUCLEARES
Design and optimization of a beam-shaping assembly for BNCT based on a neutron generator
Diseñó y optimización de un conformador del haz para terapia por captura de neutrones del boro, basado en un generador de neutrones
Fátima Padilla Cabal 1, Guido Martín Hernández 2, Arian Abrahantes Quintana 2
1Instituto Superior de Tecnologías y Ciencias Aplicadas (InSTEC)
Ave. Salvador Allende, esq. Luaces, Plaza, Ciudad de La Habana, Cuba
2Centro de Aplicaciones Tecnológicas y Desarrollo Nuclear (CEADEN)
Calle 30 No 502 e/ 5ta Ave. y 7ma. Playa, Ciudad de La Habana, Cuba
ABSTRACT
A monoenergetic neutron beam simulation study is carried out to determine the most suitable neutron energy for treatment of shallow and deep-seated brain tumors in the context of Boron Neutron Capture Therapy. Two figures-of-merit, i.e. the absorbed dose for healthy tissue and the absorbed tumor dose at a given depth in the brain are used to measure the neutron beam quality. Also irradiation time, therapeutic gain and the power generated in the target are utilized as beam assessment parameters. Moderators, reflectors and delimiters are designed and optimized to moderate the high-energy neutrons from the fusion reactions ![]() .(d;n)
.(d;n)![]() and
and ![]() (d;n)
(d;n)![]() down to a suitable energy spectrum. Metallic uranium and manganese are successfully tested for fast-to-epithermal neutron moderation as well as Fluental
down to a suitable energy spectrum. Metallic uranium and manganese are successfully tested for fast-to-epithermal neutron moderation as well as Fluental![]() for the neutron spectrum shifting. A semispherical target is proposed in order to dissipate twice the amount of power generated in the target, and decrease all the dimensions of the BSA. The cooling system of the target is also included in the calculations. Calculations are performed using the MCNP code. After the optimization of our beam-shaper a study of the dose distribution in the head had been made. The therapeutic gain is increased in 9% while the current required for one hour treatment is decreased in comparison with the trading prototypes of NG used for Boron Neutron Capture Therapy.
for the neutron spectrum shifting. A semispherical target is proposed in order to dissipate twice the amount of power generated in the target, and decrease all the dimensions of the BSA. The cooling system of the target is also included in the calculations. Calculations are performed using the MCNP code. After the optimization of our beam-shaper a study of the dose distribution in the head had been made. The therapeutic gain is increased in 9% while the current required for one hour treatment is decreased in comparison with the trading prototypes of NG used for Boron Neutron Capture Therapy.
RESUMEN
La simulación de un haz de neutrones se realizó para determinar la mejor energía de estos en el tratamiento de tumores bien profundos en la terapia por captura de neutrones del boro. Dos figuras de mérito, la máxima dosis absorbida en tejido sano y la dosis absorbida en el tumor a determinada profundidad dentro del cerebro, se utilizaron para evaluar la eficiencia del tratamiento. Se estudiaron el tiempo de irradiación, la ganancia terapéutica y la cantidad de potencia generada en el blanco, como parámetros de la calidad del haz. Se diseñaron y optimizaron moderadores, reflectores y delimitadores para moderar los neutrones de alta energía, producidos en la reacción de fusión ![]() (d;n)
(d;n)![]() , hasta un espectro de energías útiles para la terapia. Se utilizaron uranio metálico y manganeso para la moderación de neutrones rápidos a epitérmicos, mientras que el compuesto Fluental
, hasta un espectro de energías útiles para la terapia. Se utilizaron uranio metálico y manganeso para la moderación de neutrones rápidos a epitérmicos, mientras que el compuesto Fluental![]() se utilizó para el ajuste final del espectro. Se propuso un blanco semiesférico para disipar el doble de la cantidad de potencia generada en el blanco, y disminuir las dimensiones del moderador. Todos los cálculos se realizaron utilizando el código de simulación MCNP-4C. Una vez obtenida la mejor configuración del moderador, se obtuvieron las distribuciones de dosis en la cabeza y el cerebro. La ganancia terapéutica se aumentó en un 9%, a la vez que la corriente requerida para una hora de tratamiento, así como las dimensiones del moderador disminuyeron en un 50%.
se utilizó para el ajuste final del espectro. Se propuso un blanco semiesférico para disipar el doble de la cantidad de potencia generada en el blanco, y disminuir las dimensiones del moderador. Todos los cálculos se realizaron utilizando el código de simulación MCNP-4C. Una vez obtenida la mejor configuración del moderador, se obtuvieron las distribuciones de dosis en la cabeza y el cerebro. La ganancia terapéutica se aumentó en un 9%, a la vez que la corriente requerida para una hora de tratamiento, así como las dimensiones del moderador disminuyeron en un 50%.
Key words: neutron capture therapy, boron, brain, therapy, m codes, neutron generators, specifications, beam shaping, configuration, neutron beams, neutron sources, radiation dose distributions, radiation doses, simulation, accelators, neoplams
INTRODUCTION
Boron Neutron Capture Therapy (BNCT) is a binary cancer therapy modality which is very appealing due to its potential for selective cell killing. This therapy is being investigated for several types of cancers including malignant melanoma [1] and glioblastoma multiforme, a highly malignant and therapeutically persistent brain tumor, for which conventional therapies like chemotherapy, surgery, and radiotherapy are not successful. When a thermal neutron is captured by ![]() , the reaction
, the reaction ![]() (n;
(n; ![]() )
)![]() occurs, releasing two high-energy ions. Due to the high LET and RBE of these ions, only cells in close proximity to the reaction are damaged, leaving adjacent cells unaffected.
occurs, releasing two high-energy ions. Due to the high LET and RBE of these ions, only cells in close proximity to the reaction are damaged, leaving adjacent cells unaffected.
BNCT success depends on two important components. The first one is the delivery of ![]() preferentially to the tumor cells with help of tumor-seeking compounds. The second component required is a beam of thermal neutrons in the tumor cells. In practical times an epithermal neutron flux is required at the head surface. Epithermal neutrons interact with tissue components loosing energy to reach deep-seated tumors as thermal neutrons, thus increasing the probability of being captured by
preferentially to the tumor cells with help of tumor-seeking compounds. The second component required is a beam of thermal neutrons in the tumor cells. In practical times an epithermal neutron flux is required at the head surface. Epithermal neutrons interact with tissue components loosing energy to reach deep-seated tumors as thermal neutrons, thus increasing the probability of being captured by ![]() . This study has been done to design a facility for BNCT using a D-T neutron generator located at the Physics Department at CEADEN. The monoenergetic neutrons emitted by this reaction have energies of 14.1 MeV, for an incident deuteron beam with energy ranging between 100 and 400 keV [1]. The main disadvantage of the D-T reaction for BNCT is its high neutron energy of 14 MeV, which is three orders higher than that required for BNCT [2]. Moderation and shaping of the neutron beam is required for slowing down neutrons coming from the source, as well as filtering/removing fast and thermal neutrons undesirable for the therapy.
. This study has been done to design a facility for BNCT using a D-T neutron generator located at the Physics Department at CEADEN. The monoenergetic neutrons emitted by this reaction have energies of 14.1 MeV, for an incident deuteron beam with energy ranging between 100 and 400 keV [1]. The main disadvantage of the D-T reaction for BNCT is its high neutron energy of 14 MeV, which is three orders higher than that required for BNCT [2]. Moderation and shaping of the neutron beam is required for slowing down neutrons coming from the source, as well as filtering/removing fast and thermal neutrons undesirable for the therapy.
Gas-to-metal targets that are commonly used in D-T neutron generators, can tolerate temperatures up to 240oC. Beyond that temperature, tritium atoms start to be released from the metal, that’s why a cooling system of the target must be included in all the calculations.
Accepted figures-of-merit used to measure the neutron beam quality are based on biological criteria. One of the figures-of-merit used in our study is the dose distribution in the cells of the brain at different depths, and the therapeutic gain obtained for it. These doses are limited by Brookhaven National Laboratory’s (BNL) clinical trial protocol [3], which specifies that the local absorbed dose to the healthy tissues must not exceed 12.5 Gy-equivalent anywhere in the brain. As another constraint for this radiation therapy is the treatment time, the irradiation time was also study. For the comfort of the patient, a five hour treatment is undesirable.
In this work, we present a study to determine the final configuration of a shaper for the beam to produce epithermal neutron beams for the treatment of deep-seated brain tumors considering the D-T fusion reaction as the primary source of neutrons, based in some previous results obtained by Martin [4]. The materials used for moderators and reflectors which offers the most suitable results for therapeutically useful regions were obtained previously, and were set in this study. A geometrical configuration was optimized for our shaping assembly.
Figures of merit for BNCT
For deep seated tumors, in order to deliver thermal neutrons to them, was found in the literature that we need to supply epithermal neutrons with an energy distribution peaking around 10 keV. For lower energy, neutrons will not reach this zone in the brain, the contribution to the dose at the center of the brain is less significant.
For the calculations of the absorbed doses, a method described by Martin [5] was used. It’s briefly described in the following paragraph. For a volume irradiated by a parallel neutron beam, with a neutron energy spectrum p(Ei), the dose at any point in the beam centerline is calculated from:
![]()
where d(r, ![]() )is the RBE-weighted absorbed dose delivered to a small volume located at r. The space-energy dependent magnitude d(r,
)is the RBE-weighted absorbed dose delivered to a small volume located at r. The space-energy dependent magnitude d(r, ![]() )is a data matrix, previously calculated, containing dose per position per energy and that is used to calculate the dose to the head and consequently, the therapeutic gain of the beam. The latter is defined as the dose to tumor divided by the maximum dose to healthy tissue. The therapeutic gain and the irradiation time are the parameters that determine the quality of a neutron beam for BNCT.
)is a data matrix, previously calculated, containing dose per position per energy and that is used to calculate the dose to the head and consequently, the therapeutic gain of the beam. The latter is defined as the dose to tumor divided by the maximum dose to healthy tissue. The therapeutic gain and the irradiation time are the parameters that determine the quality of a neutron beam for BNCT.
The Snyder’s head model [6] modified by Liu et al. [7] is used. The elemental composition and densities in the scalp, skull, and the brain are taken from Brooks et al. [8]. The head is irradiated bilaterally, from ear to ear, to treat a centrally located 1-![]() -volume tumor, at 73 mm from the skin. The tumor is loaded with 45 ppm by mass of
-volume tumor, at 73 mm from the skin. The tumor is loaded with 45 ppm by mass of ![]() and healthy tissue is loaded with 15 ppm [9]. The boron is explicitly added to the elemental composition of the tissue to account for boron self-shielding effects. To calculate dose distribution in the head tissues, the head geometry is divided into cubic elements (lattice) of 1
and healthy tissue is loaded with 15 ppm [9]. The boron is explicitly added to the elemental composition of the tissue to account for boron self-shielding effects. To calculate dose distribution in the head tissues, the head geometry is divided into cubic elements (lattice) of 1 ![]() volume. The lattice cells are the elementary volumes to tally the energy deposits for tracked neutrons and photons. Biologically weighted doses are calculated from physical doses as:
volume. The lattice cells are the elementary volumes to tally the energy deposits for tracked neutrons and photons. Biologically weighted doses are calculated from physical doses as:
![]()
where the subscripts represent each material component, i.e. H and N for hydrogen and nitrogen, ![]() for boron, and g for photon. Compound biological effectiveness (CBE), which represents a convolution of the
for boron, and g for photon. Compound biological effectiveness (CBE), which represents a convolution of the ![]() microdistribution with the radiobiological effects (RBE of the reaction products) of the thermal neutron fluence, is 1.3 for healthy tissue and 3.8 for tumor. Blue et al. [10] present a relation between the neutron RBE and neutron energy appropriate for BNCT, using an empirical RBE-LET relationship.
microdistribution with the radiobiological effects (RBE of the reaction products) of the thermal neutron fluence, is 1.3 for healthy tissue and 3.8 for tumor. Blue et al. [10] present a relation between the neutron RBE and neutron energy appropriate for BNCT, using an empirical RBE-LET relationship.
MATERIALS AND METHODS
The main challenge for shaping the beam is to decrease the neutron energy in three order of magnitude, at the time that the economy of neutrons. Shifting neutron energies from fast to epithermal by means of neutron interaction with a set of materials is technically possible, but a large number of neutron collisions are required. As a result, the neutron flux decreases due to neutron capture, neutron escape from the geometry and the ![]() law.
law.
For the D-T generator a semispherical target is proposed. The target calculated is a Ti-T target, with a proportion of 1.6 atoms of tritium per atom of titanium. The advantage of this type of target, is that for a beam radius the effective area is twice the area of a flat target. To dissipate the amount of power generated in the target, as soon as we increase the effective area in a factor of 2, the power density will decrease in the same factor. A first configuration is proposed based in the previous work developed by Martin [4]. The proposed design is shown in figure 1.

The first cell is a sphere of 14 cm of radius, the second one another sphere of 36 cm of radius of manganese or some alloy with copper or cobalt, and the third one is a rectangular cavity of 12 cm of Fluental![]() , a compound of
, a compound of ![]() , Al and LiF. Besides Lead is used as a lateral and back reflector for neutrons to keep the flux as invariable as it´s possible and nickel for scatter the neutrons with energies unsuitable.
, Al and LiF. Besides Lead is used as a lateral and back reflector for neutrons to keep the flux as invariable as it´s possible and nickel for scatter the neutrons with energies unsuitable.
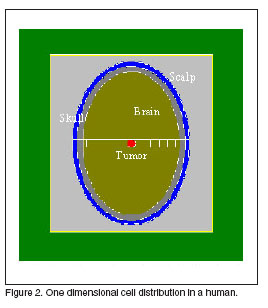
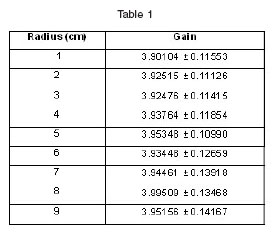
The beam radius was range between 1 cm and 9 cm. The first group of calculations were made for a one-dimensional analysis dose distribution in the head. A tumor of 1![]() was located in the centre of the brain at 73 mm from the head surface as is shown in figure 2. The therapeutic gain was the figure of merit used to evaluate the moderator. The results are displayed in the table 1, showing not so much difference for the therapeutic gain in all cases.
was located in the centre of the brain at 73 mm from the head surface as is shown in figure 2. The therapeutic gain was the figure of merit used to evaluate the moderator. The results are displayed in the table 1, showing not so much difference for the therapeutic gain in all cases.
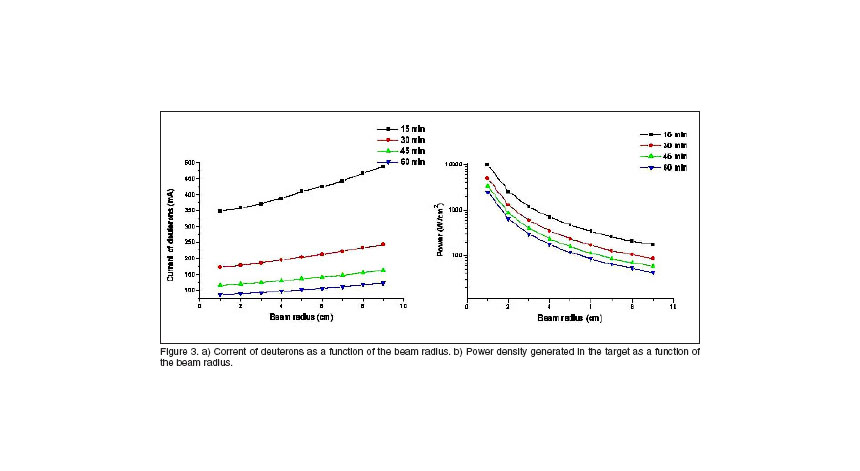
As the therapeutic gain is a parameter which remains constant with the beam radius, a second group of calculations of the current of deuterons required and power dissipated in the target for different irradiation times were done. For small radius our generator will be more compact and of course more suitable to build.
From figure 3, we can see that the limit in the decreasing of the beam radius is the dissipation of the heat generated in the target. A construction of the most efficient cooling system will offer the optimal size of the beam-shaper. In line with this work, a cooling system with a winglet structure was developed, and it’s shown in figure 4. The winglets are built in copper, the water is used as dissipater, and the structure is surrounded by a semispherical layer of aluminium.
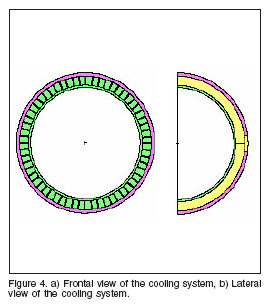
To study the influence of the cooling system in the moderation process for neutrons, the neutron spectrum after the cooling system was evaluated for three different configurations, a) using for the transport of neutrons the real winglet structure of the system, b) using an average material of copper and water, and c) with no cooling system in the target. The results can be found in figure 5.

Once the cooling system is included in the beam shaper, an optimization of the distances in the longitudinal and vertical dimensions had been done. From the technical limit in the construction of the cooling system, the smallest radius that we can reach for the beam is 2.5 cm.
For the moderation of neutrons, at the moment that the primary source is defined, there are three factors involved in the process of designing a shaper: a) materials, b) dimensions and c) geometry. The first one of these factors will not be taking into account, as was explained before. Transporting neutrons throughout a simple geometry model does the former. An isotropic source of 14 MeV neutrons is placed at the semispherical target, center of a sphere containing the material to be tested. Either the mean neutron energy or the therapeutic gain of the beam and the fluence (![]() ; fraction of emitted neutron by source neutron) are studied at the surface of the sphere depending on its radius. To perform those calculations, the general-purpose code MCNP 4
; fraction of emitted neutron by source neutron) are studied at the surface of the sphere depending on its radius. To perform those calculations, the general-purpose code MCNP 4![]() is used.
is used.
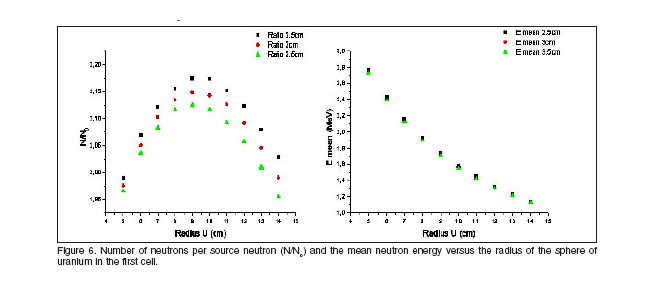
The first material which neutrons will interact with, should have high scattering cross section and low capture cross section for fast neutrons. The function of the first cell is to quickly convert the 14 MeV peak into a softer spectrum. In addition, this material should produce neutrons by (n,2n) reaction or fission to maintain constant the magnitude ![]() or even increase it with the distance. Uranium is the best choice for this cell. Figure 6 shows the calculated number of neutrons per source neutron (
or even increase it with the distance. Uranium is the best choice for this cell. Figure 6 shows the calculated number of neutrons per source neutron (![]() ) and the mean neutron energy versus the radius of the sphere of uranium in the first cell. The best radius of uranium is nearby 9 cm, for higher values the neutrons starts to be absorbed in the cell.
) and the mean neutron energy versus the radius of the sphere of uranium in the first cell. The best radius of uranium is nearby 9 cm, for higher values the neutrons starts to be absorbed in the cell.
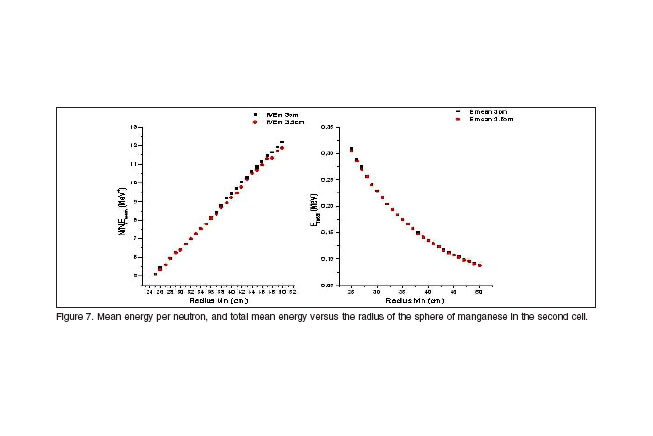
Once the first cell dimension is chosen, a second cell is added to the first one and the mean energy and mean energy per neutron of the beam emerging from the sphere are newly calculated for different radii. The function of this second cell is to shift the still hard neutron spectrum to energies close to a few hundreds of keV. In order to produce such energy lost without broadening the energy spectrum and simultaneously achieving a minimum cell thickness, the material with best perspectives is manganese. Results are displayed in figure 7. As the mean energy per neutron is an increasing function with the distance, for wider thickness of manganese, the results are more attractive. On the other hand, for more than 40 cm of manganese thickness the mean energy of neutrons is more and less in the same order, that´s the reason for which including more manganese is not useful anymore. The second cell will have a thickness between 40 cm and 50 cm of manganese.
To finally moderate the energy spectrum emerging from the second cell to energies useful for BNCT a third cell is added. A different approach is used for this cell. Instead of studying the mean neutron energy, the therapeutic gain of the beam is investigated. Fluental![]() is added as a third material for a final shifting of the spectrum. Calculations were done for a beam radius of 3 cm, and 40 cm, 45 cm and 50 cm of manganese in the second cell. Results of the therapeutic gain and its product with the neutron fluency are pointed out in figure 8. The higher values of the therapeutic gain are obtained for 50 cm of manganese, and 18 cm of Fluental
is added as a third material for a final shifting of the spectrum. Calculations were done for a beam radius of 3 cm, and 40 cm, 45 cm and 50 cm of manganese in the second cell. Results of the therapeutic gain and its product with the neutron fluency are pointed out in figure 8. The higher values of the therapeutic gain are obtained for 50 cm of manganese, and 18 cm of Fluental![]() , and all the longitudinal dimensions for the assembly had been set.
, and all the longitudinal dimensions for the assembly had been set.

After defining the composition and an estimate of the Beam Shaping Assembly linear dimensions (9 cm uranium + 50 cm manganese + 18 cm Fluental![]() ) the geometry design is accomplished. Designing the geometry can be done taking care of the physics involved in shaping the neutron spectrum. In order to save the amount of neutrons for the therapy, the geometry proposed is shown in figure 9.
) the geometry design is accomplished. Designing the geometry can be done taking care of the physics involved in shaping the neutron spectrum. In order to save the amount of neutrons for the therapy, the geometry proposed is shown in figure 9.

Lead is included not only as lateral and back reflector, also for the transport and moderation of the neutrons in the beam direction. The absorption cross section for Lead in the epithermal range is much smaller than for manganese, so the including of the Lead cone will warranty that more neutrons reach the head surface. Besides, is also used as gamma shielding in the entire assembly. Three more parameters must be optimized, a) the angle of the manganese cone, b) the Fluental![]() height for the irradiation cavity, c) the total height of Lead for the moderator. The therapeutic gain was study as the beam assessment parameter for all the cases.
height for the irradiation cavity, c) the total height of Lead for the moderator. The therapeutic gain was study as the beam assessment parameter for all the cases.
A first group of calculations were done for different angles of the cone, (30º, 45º and 60º) and also, as a function of the height of Fluental![]() for the cavity. Results can be found in figure 10. Here we can design our geometry with 45º of the angle of manganese and 18 cm of Fluental
for the cavity. Results can be found in figure 10. Here we can design our geometry with 45º of the angle of manganese and 18 cm of Fluental![]() , which is the configuration with better results.
, which is the configuration with better results.
The optimization for the last parameter of the geometrical design was done estimating the product of the neutron fluency and the therapeutic gain for the treatment as a function of the height of Lead as back reflector. With these calculations, which results are displayed in figure 10, the final height of the shaping assembly was set to 100 cm.

The configuration obtained offers the best results for the therapy, but in order to evaluate the real assessment parameters, a complete mapping of the dose in the entire head must be done. The neutron and gamma fluxes in the head were calculated for the moderator. The neutron flux (1.25214 x ![]() ) is higher than the gamma flux (9.5587 x
) is higher than the gamma flux (9.5587 x ![]() ) but only 13 times, so the gamma influence on the dose received is significant. All the following simulations were made considering the effect of the gamma flux associated to the neutron flux. In fact, the dose caused due to the gamma and neutrons were determined and both have the same order of magnitude.
) but only 13 times, so the gamma influence on the dose received is significant. All the following simulations were made considering the effect of the gamma flux associated to the neutron flux. In fact, the dose caused due to the gamma and neutrons were determined and both have the same order of magnitude.
The gamma flux is important to taking into care in the optimization of the therapy. The effective dose in all the head tissues will be an addition of the corresponding dose caused because of the neutron flux (generated in the interaction with B, N, and H) and also because of the gamma flux. The photons are generated in all places when neutrons interact in all the materials of the beam-shaper. Two new calculations were performed in order to have an idea of the influence in the head dose distribution of considering the generation of photons only in the head in relation with the real case, photons generated in all the places of the moderator. The results are shown below in table 2.

Neutron dose is the same for both cases, the only modification introduced was the importance of considering transport of photons in two different places in the beam shaper, the neutron flux remains constant. Nevertheless the gamma flux increase in values between 28%-33%, that´s why we consider that the production of photons have to be considered in all the places of the moderator.
With the last results, the dose distribution for all the head was mapped. The head was divided in elemental cells of 1 cm3 and the effective doses in each one which was filled with more than the 10% of its volume were calculated. Results are shown in figure 11. The maximal dose in healthy tissue is less than 30% the dose in the tumor, in this case we obtain a therapeutic gain of 3.4. The statistical errors in these cases are very high. The cells in the head surface, filled with a very small percent of tissue, require a large amount of time calculation to remove the statistical peaks. Outside the brain zone, in which the errors are in the order of the 5%, the errors have values of even 25%, which are undesirable for our calculation. In the tissues of the scalp and the skull as the occurrence of events is very small, the dose in these zones is not significant compared with the dose in the brain, that´s why a last simulation was done, considering a mapping in only cells of the brain. This result is shown in figure 12.
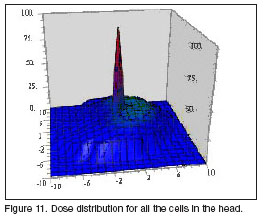

The therapeutic gain in this case is 3.44, very similar to the former simulation but the statistical errors were decreased to less than 5% in the brain zone and 15% in the head surface. From these results we consider the idea of making future simulations with the mapping only in the brain, it´s possible to reduce the statistical errors in 10% in the head surface. Even when we are not considering the dose in the scalp and skull they can only represent a 7% of the dose in the tissues nearby the tumor in the brain.
In both cases the dose distribution obtained for bilateral irradiation is not symmetrical in the XZ or YZ planes while is symmetrical in the XY planes. This behaviour is related with the fact that the bilateral irradiation only inverted the coordinates in X and Y axis, keeping the Z coordinates invariables.
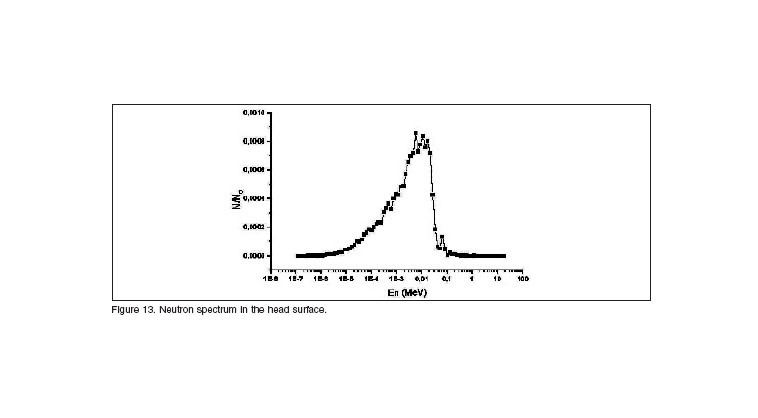
The last assessment parameter evaluated was the neutron spectrum in the head surface using our moderator. The spectrum obtained, displayed in figure 13, is in complete agreement with the results get form literature, showing a peak nearby 10 keV, and filtering efficiently the fast and thermal component form the beam. This result, and the therapeutic gain obtain of 3.45 endorse our design of the beam shaping assembly for neutron capture therapy.
CONCLUSIONS
Based on our previous study on the quality of neutron beams for BNCT, the deuterium-tritium fusion reaction has been exercised to design a beam shaping assembly. A semispherical target is proposed for the D-T generator, in order to increase the effective area of the target, and make more compact our facility.
For this neutron source, a final assembly has been design with an uranium cell of 9 cm, a manganese cell of 50 cm in a conic shape with 45º of amplitude, an irradiation cavity surrounded by 18 cm of Fluental![]() and 100 cm of lead as back reflector for neutrons.
and 100 cm of lead as back reflector for neutrons.
The geometry and size of the beam-shaping assembly considerably influence on the quality of the neutron beam, mainly on the number of neutron at the beam port per source neutron. According to our calculation, a substantial improvement in the treatment features of a BNCT facility based on the D-T reaction is obtained. A complete dose distribution in the head cells, and a neutron spectrum endorses our beam shaping assembly for using in BNCT facilities.
RECOMMENDATIONS
This work was direct to demonstrate the possibility of installation a BNCT facility using the D-T generator located at CEADEN. All the calculations were done using the MCNP-4C code, and the evaluation of the assembly has been done since trading commercial prototypes for NCT, but some experimental verification of some parameters is unsettled until now.
REFERENCES
1. BINELLO E, SHORTKRO S, JONES A, VIVEIROS C, LY A, SLEDGE CB, DAVISON A, SHEFER RE, YANCH JC. Research in Boron Neutron Capture Synovectomy. Proceedings of the International Conference on Neutrons in Research and Industry. Crete, 1996.
2. VERBEKE JM, LEE Y, VUJIC J, LEUNG KN. Compact accelerator and beam shaping assembly design for BNCT based on the D-T fusion reaction.
3. NIAS AHW. An Introduction to Radiobiology. New York: John Wiley & Sons, 1990.
4. MARTIN G, ABRANTES A. A conceptual design of a beam shaping assembly for BNCT based on DT neutron generators. Med Phys. 2004;( 31).
5. MARTIN G. A method for fast evaluation of neutron spectra for BNCT based on in-phantom figure-of-merit calculation. Med. Phys. 2003; 30 (3).
6. SNYDER WS, FORD MR, WAGNER GG, FISHER HL. Estimates of absorbed fractions for monoenergetic photon sources uniformly distributed in various organs of heterogeneous phantom MIRD. J. Nucl. Med. 1978; (Suppl. 3, Pamphlet 5).
7. LIU HB, GREENBERG DD, CAPALA J, WHEELER FJ. An improved neutron collimator for brain tumor irradiations in clinical boron neutron capture therapy. Med. Phys. 1996; (23).
8. BROOKS RA, DICHIRO G, KELLER MR. Explanation of cerebral white-gray contrast in computed tomography. J. Comput. Assist. Tomogr. 1980; (4).
9. SUNG-JOON YE. Boron self-shielding effects on dose delivery of neutron capture therapy using epithermal beam and boronophenylalanine. Med. Phys. 1999; (26).
10. BLUE TE, WOLLARD JE, GUPTA N, GRESKOVICH JF. An expression for the RBE of neutrons as a function of neutron energy . Phys. Med. Biol. 1995; (40).
Recibido: 12 abril de 2007
Aceptado: 28 mayo de 2007
1Instituto Superior de Tecnologías y Ciencias Aplicadas (InSTEC)
Ave. Salvador Allende, esq. Luaces, Plaza, Ciudad de La Habana, Cuba
2Centro de Aplicaciones Tecnológicas y Desarrollo Nuclear (CEADEN)
Calle 30 No 502 e/ 5ta Ave. y 7ma. Playa, Ciudad de La Habana, Cuba













