Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Nucleus
versión impresa ISSN 0864-084X
Nucleus n.42 Ciudad de La Habana jul.-dic. 2007
CIENCIAS NUCLEARES
Raman spectroscopy of poly (3-hydroxybutryrate) modified with poly (vinyl acetate) by radiation-induced copolymerisation
Espectroscopía raman del poly (3-hidroxibutirato), modificadi con poli (acetato de vinilo) por copolimerización radio-inducida
Maykel González Torres1, Norma Galego Fernández1,2, Pedro Ortiz del Toro2, Manuel Rapado Paneque3
1Instituto de Ciencia y Tecnología de Materiales (IMRE), Universidad de la Habana, Cuba
2Facultad de Química, Departamento de Química-Física, Universidad de la Habana, Cuba
3Centro de Aplicaciones Tecnológicas y Desarrollo Nuclear (CEADEN), Calle 30 No. 502 e/ 5ta
Ave. y calle 7ma, Playa, Ciudad de La Habana, Cuba
norma@imre.oc.uh.cu, mglez@imre.oc.uh.cu, rapado@ceaden.edu.cu
ABSTRACT
Poly (3-hydroxybutyrate) (PHB) is an important material used in the field of medicine. However in common conditions, PHB has some disadvantages. It is very brittle and slightly hydrophobic polymer. This somewhat limit its applications. Radiation can be used to improve its chemical properties. In the present study, the substrate, modified by radiation-induced graft copolymerization with vinyl acetate (VAc), was characterized using FTIR (Fourier Transform Infra-red) and Raman spectroscopy. FTIR spectroscopy did not reveal any significant bands but Raman spectroscopy revealed the formation of new bands that characterize the new material.
RESUMEN
El poli (3-hidroxibutirato) (PHB) es un material importante de aplicación en el campo biomédico. Sin embargo, en condiciones comunes, se presenta con algunas desventajas que limitan su aplicación, como son su fragilidad y su moderada hidrofobicidad. Debido a esto, su campo de aplicaciones se encuentra limitado. Las radiaciones se pueden utilizar para transformar químicamente este sustrato y mejorar sus propiedades. En este estudio, el PHB modificado con el acetato de vinilo (VAc) por medio de una copolimerización por injerto radio-inducido, fue caracterizado utilizando espectroscopías FTIR y Raman. El análisis por FTIR no reveló ninguna banda característica que diferenciara al copolímero por injerto de los homopolímeros que lo forman, pero la espectroscopía Raman reveló la formación de nuevas bandas que caracterizan al nuevo material.
Key words: polymers, graft polymers, gamma radiation, raman spectroscopy, radiation doses
INTRODUCTION
Graft copolymerization is a well known method for the modification of chemical structure of polymers. The typical method of the graft polymerization is a radical polymerization of polar monomers onto non-polar substrates. This method is used in order to modify the physical and chemical properties of the base polymer and extent its limited applicability [1] In addition, the radiation-induced graft polymerization is shown to be one useful technique to modify biomaterials such as PHB. This can be explained, because it does not need any catalyst or initiator. Graft copolymers with high purity under low temperature can be also obtained. Therefore, many advantages compared to graft copolymerization with chemical initiators can be found [2]. Besides, the PHB is an important material used in biomedical applications, such as drug release and bone tissue engineering, due to its high biocompatibility, non oral toxicity, and entire biodegradability [3-5]. But under the common conditions, this natural occurring polyester produced by microorganisms is slightly hydrophobic plastic. It is also very brittle and thermal unstable material about the melting temperature [6]. This is the reason why, many efforts have been performed in order to improve these properties, using gamma radiation-induced graft copolymerization of various monomers onto it [7-12]. Of the possible chemical modifications on PHB, graft copolymerization by gamma radiation- induced reactions is attractive to expand applications as functional materials. Some of poly (3-hydroxybutyrate)-graft modifications have appeared in the literature by the use of oxygen plasma treatment [13], perfluoro hexane plasma [14] and ammonia plasma [15]. However, the chemical modifications were only observed on the material surface. In this sense, gamma irradiation is well known by its advantages. For the reason that, one advantage of using gamma irradiation induced grafting rather than the others mentioned treatments are the ability to penetrate and modify into the material 3D scaffold [16].
PHB have been previously grafted with acrylic acid (AA) [7, 11, 12], methyl methacrylate (MMA) [7, 8], 2- hydroxy-ethyl methacrylate (HEMA) [8], styrene (St) [9] and isoprene (Ip) [10], with the purpose to improve PHB chemical properties .The materials obtained were also studied by different physicochemical techniques.
In this work, the authors used PHB-graft-PVAc, obtained by radiation-induced graft copolymerization on VAc onto PHB [17] and studied the new material using FTIR and Raman spectroscopes. The spectral analyses have confirmed the presence of a new material.
MATERIALS AND METHODS
PHB-graft-PVAc was obtained by the authors by radiation-induced graft copolymerization of VAc onto PHB [17]. The material characterization revealed that the grafting degree was 43,5% and the grafting index 25% determined by gravimetric technique and 13C-RMN spectroscopy [17].
Characterization
FTIR spectroscopy
The FTIR spectra of the samples were recorded with Jasco FT/IR- 460 Plus (Jasco Instrument Corp., Japan) equipment. Milligrams of the sample were used in solid form. In the data collection 64 scans, at 2 cm-1 of resolution, to scan rate of 2 mm/ s and a gain of 4 were used.
Surface-enhanced Raman spectroscopy (SERS)
All SERS spectra were collected with a Renishaw Invia system, equipped with Peltier cooled CCD detectors (-70°C). Excitation lines of 514.5, 633, and 785nm were used. Spectra were collected in Renishaw’s extended collection mode, by co-adding three scans with accumulation times of 10s.
RESULTS AND DISCUSSIONS
Figure 1 shows the radiation-induced graft copolymerization reaction. The simultaneous irradiation technique with a 60Co gamma rays source was used. The dose rate was 1. 57 kGy/h and the dose 10 kGy at room temperature. The reaction was carried out in bulk with a m (VAc)/m (PHB) = 6. The studied material is the product of this reaction.
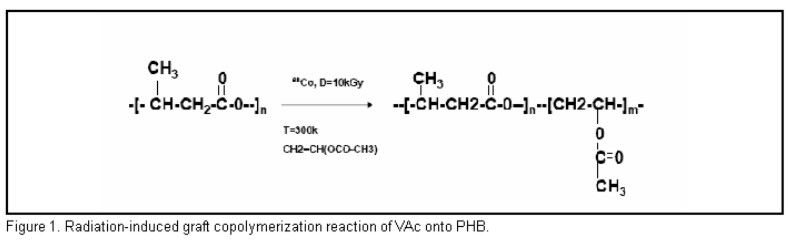
Infra-red investigations
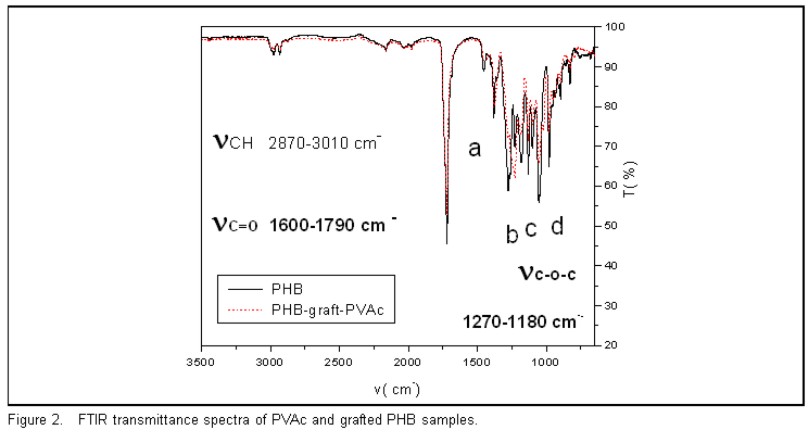
Spectroscopic infrared techniques were used to verify vinyl acetate grafting to the PHB sample. FTIR transmittance spectra of PVAc and grafted PHB sample are shown in Figure 2 (700-3500 cm-1 region). Because of the spectra was recorded in the transmittance mode, peaks in the 1000-1400 cm-1 region are saturated. ATR-FTIR spectra run on the same samples did not reveal any significant differences in the bands in this region because vinyl acetate and PHB have the same functional groups. This explained why different bands were not observed, but the contribution of different physical states. The FTIR transmittance spectrum samples only showed an aliphatic CH stretch at 2870-3010 cm-1,a C = O stretch at 1600-1790 cm-1, and differences in band intensities in the C-O-C stretch (1270-1180 cm-). In addition, the bands at 1185 cm-1 (d in figure 2), 1228 cm-1 (c in figure 2), 1279 cm-1 (b in figure 2) and the CH3 at 1382 cm-1 (a in figure 2) are useful for qualitative analysis of the cristallinity degree. This analysis is done in other work [17].
Raman results
The structure of the PHB-graft-PVAc obtained by the radiation-induced graft copolymerization reaction was investigated by using Raman spectroscopy. As a result of the copolymerization reaction any difference must be detected by the Raman spectrometer as effect of a new material formation.
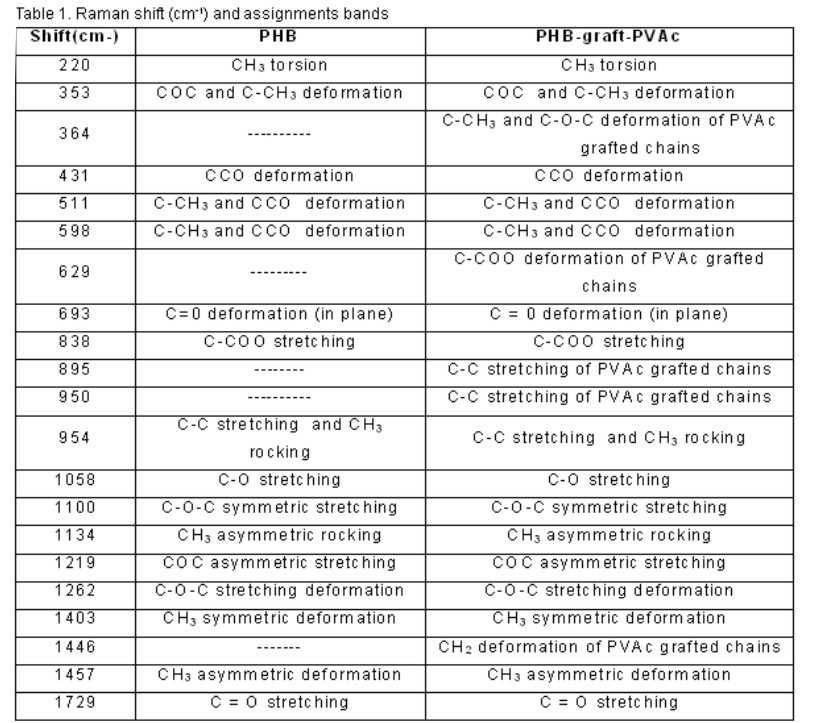
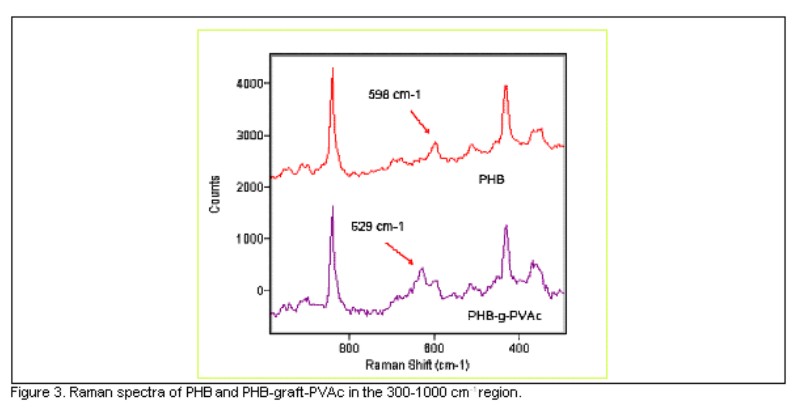
Figure 3 shows the 300-1000 cm-1 region of the Raman spectra of a) PHB and b) PHB-graft-PVAc. The fact that the 598 cm-1 frequency corresponding to the coupling mode of C-CH3 and CCO deformation is common to both spectra can be seen clearly. Whereas, the graft copolymer spectra reveals at 629 cm-1 a new and well separated band corresponding to the couple mode of C-COO deformation of PVAc grafted chains. These results have demonstrated that the radiation-induced graft copolymerization of VAc onto PHB was achieved. Other bands to corroborate the PHB-graft-PVAc formation are shown in table 1.
It is important to explain here that the Raman investigation was done in order to demonstrate the graft copolymer formation by means of finding new bands related with the synthesised material or signals related with the quaternary substituted carbon that could not be observed by the NMR spectroscopy. Unfortunately this latter was not either found, but the former was seriously studied.
The band assignments for Raman spectra of PHB and PHB-graft-PVAc are shown in table 1. Unlike other graft copolymers, an observed difficulty was the fact that PHB and PVAc have the same functional group.
Moreover the monomer units have the same molecular weight (86 g/mol). Thereby, the bands assignments were based in the corresponding comparison of the FTIR spectra and the Raman investigations of PHB and PVAc studied by other authors [18, 19].
Table 1 lists the Raman shift (cm-1) and assignments of the Raman bands of PHB and PHB-graft-PVAc. The bands at 1729, 1457, 1403 and 1262 cm-1 in the Raman spectra of the PHB and the graft copolymer may be assigned to C = O stretching mode, CH3 asymmetric deformation, CH3 symmetric deformation and C-O-C stretching deformation respectively.
Bands at 1219, 1134, 1100 and 1058 cm-1 are assigned, respectively, to COC asymmetric stretching, CH3 asymmetric rocking, C-O-C symmetric stretching and C-O stretching.
In the 220-954 cm-1 region , the bands at 954, 838, 693, 598, 511, 431, 353 and 220 cm-1 like common Raman signals of PHB and PHB-graft-PVAc can be seen clearly .The three first ones corresponding to C-C stretching and CH3 rocking, C-COO stretching and C=0 deformation (in plane) respectively. The two subsequent bands correspond to C-CH3 and CCO deformation and the three last ones corresponding to CCO deformation, COC and C-CH3 deformation, and CH3 torsion.
Therefore, the appearance of bands at 364, 629, 895, 950 and 1446 cm-1 are attributed to the graft copolymer, and strongly corroborates the PHB-graft-PVAc formation. There is a clear difference in the mentioned bands in the graft copolymer compared to the control PHB Raman spectra. Band at 629 cm-1 was above shown and discussed (figure 3). The assignments for the rest is the CH2 deformation of PVAc grafted chains at 1446 cm-1 ,the C-C stretching of PVAc grafted chains at 950 and 895 cm-1, and the C-CH3 as well as C-O-C deformation of PVAc grafted chains at 364 cm-1. From this finding the presence of a new material was confirmed. In spite of the efforts, the asymmetric carbon was not detected, but is of note the great correspondence between the different spectroscopic techniques used to characterize the material.
CONCLUSIONS
FTIR and Raman spectroscopic techniques were used to verify vinyl acetate grafting to the PHB sample. Significant differences at the bands in the FTIR spectra were not observed, because of vinyl acetate and PHB have the same functional groups, but the contribution of different physical states. However, Raman studies revealed the formation of new bands. For instance, the graft copolymer Raman spectra revealed at 629 cm-1 a new and well separated band corresponding to the couple mode of C-COO deformation of PVAc grafted chains. These results have demonstrated that the PHB substrate was modified by radiation- induced graft copolymerization with vinyl acetate and PHB-graft-PVAc formation was achieved.
The authors are grateful to: Joaquin Iglesias and Sonia Altanés for their technical assistance.
REFERENCES
[1]- Mahmoud Nasefa , Mohamed, El-Sayed Ahmed Hegazyb, Prog. Polym. Sci, 29 (2004)499.
[2]- HOFFMAN, A.S., Advanced radiation chemistry research: current status. ISSN 1011-4289.Viena (1995).
[3]- HASIRCI, V., et al., J. of Biotechnology, 86 (2001)135.
[4]- GURSEL, I., et al., J. Microencapsulation, 19 (2)(2002)153.
[5]- BARON, E. T., et al., J. Microencapsulation, 19(3) (2002)363.
[6]- LUPKE, T., METZNER, J., Macromol Symp, 127 (1998)227.
[7]- MITOMO, H. , Enjôji, Pure Appl. Chem., A32 (3) (1995), 429.
[8]- BAHARI, K., MITOMO, H., ENJOJI, T. Die Angewandte Makromolekulare Chemie, 250 (4352) (1997)31.
[9]- MITOMO, H., WATANABE, Y., YOSHIF, F. , MAKUUCHF, K., Radiat. Phys. Chem., 46(2) (1995) 233.
[10]- JIANG, T., HU, P., Polymer Journal, 33(9) (2001)647.
[11]- GRØNDAHL L., CHANDLER-TEMPLE A. , TRAU M., Biomacromolecules (2005)2197.
[12]- YUKI, W., HIROSHI, M., et al., J Appl Polym Sci 101 (2006)3856.
[13]- MAS, A., JAABA, H., Schue, F. Macromol. Chem. Phys., 197 (1996)2331.
[14]- FLOSCH, D., CLAROTTI, G., GECKELER, K. E., SCHUE, F., GOPEL, W. J., Membrane Sci., 73 (1992)163.
[15]- NITSCHKE, M., SCHMACK G., JANKE A., SIMON, F., PLEUl, Werner, D.; C. J. Biomed. Mater. Res., 59 (2002)632.
[16]- Trudy Pomerantz Carswell , Hill David J.T . Radiat. Phys.Chem. 45 (5) (1995)737.
[17]- Submitted to Polymer International.
[18]- SIMOPU, A., MUCKLICH, M., KUNATH, D. und g. Heixtz. Journal of Polymer Science Vol. XXX Prague symposium (1958)201.
[19]- Furukawa Tsuyoshi , Sato Harumi , Murakami Rumi , Zhang Jianming , Noda Isao , Ochiai Shukichi , Ozaki Yukihiro, Polymer 47 (2006)3132.
Recibido: 27 de abril de 2007
Aceptado: 1 de noviembre de 2007
norma@imre.oc.uh.cu, mglez@imre.oc.uh.cu, rapado@ceaden.edu.cu












 Curriculum ScienTI
Curriculum ScienTI
