Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Nucleus
versión impresa ISSN 0864-084X
Nucleus n.42 Ciudad de La Habana jul.-dic. 2007
CIENCIAS NUCLEARES
Standard test of labelling for efficiency for quality control of no carrier added 90YCL3
Prueba estándar de la eficiencia del marcaje para el control de la calidad del 90YCI3 sin portador añadido
Milos Beran, K. Eigner Henke, J. Srank, F. Melichar
Department of Radiopharmaceuticals
The Institute of Nuclear Physics, The Academy of Sciences of the Czech Republic (INP ASCR)
Rez near Prague, Czech Republic
milber@attas.cz, beran@ujf.cas.cz
ABSTRACT
Particle emitting radionuclides (e.g. b--emitters 90Y and 177Lu, b-emitter 149Tb, Auger electron emitter 165Er or positron emitter 86Y) are more and more frequently used in research and clinical practice for imaging and radionuclide targeted therapy in nuclear medicine. These radiometals, altogether threevalent lanthanides or actinides with high specific radioactivity, coupled to biomolecule carriers (peptides or monoclonal antibodies) through chelating link (e.g. DTPA or DOTA) bind to specific antigens and/or receptors of diseased tissues, which enables the imaging (positron emitters) or destruction (b--, a-, and Auger electron emitter) of the diseased tissue releasing the antigens or carrying the receptors. The radionuclide precursor 90YCl3 (solution of hard b--emitter 90Y in diluted HCl) with high purity and specific activity is already commercially produced and succesfully used in nuclear medicine, e.g. for radioimmunotherapy (RIT) of Lymphoma. Specification and purity of our product obtained using extraction 90Sr/90Y generator (using technology of centrifuge extractors with di-2-ethylhexylphosphoric acid, D2EHPA) is examined and compared to other similar products in this contribution. A standard method for determination of labelling efficiency of the 90YCl3 precursor based on its reaction with DOTA-Tyr3-Octreotide (DOTA-TOC) and ITLC-SG chromatographic separation is described and proposed for the quality control.
RESUMEN
Los radionucleidos emisores de partículas (ej. emisores b 90Y y 177Lu, emisores a 149Tb, emisor de electrones Auger 165Er o emisor de positrones 86Y) se emplean cada vez más en la investigación, en la práctica clínica para el procesamiento de imágenes y en la terapia dirigida de radionuclidos en medicina nuclear. Estos radiometales junto con actínidos o lantánidos trivalentes con alta actividad específica, asociados a portadores de biomoléculas (péptidos o anticuerpos monoclonales) por medio de un enlace con quelatos (ej. DTPA o DOTA) se unen a antígenos específicos receptores de tejidos afectados que permiten el procesamiento de imágenes (emisores de positrones) o la destrucción (b--, a-, y emisores de electrones de Auger) de los tejidos afectados que liberan los antígenos o portan los receptores. El precursor del radionucleido 90YCI3 (solución de emisor b- duro - 90Y en HCI diluido) con elevada pureza y actividad específica ya se produce comercialmente y se usa exitosamente en la medicina nuclear, por ejemplo, para la radioinmunoterapia (RIT) del linfoma. En esta contribución se examina la especificación y la pureza de nuestro producto, obtenido mediante extracción con generador 90Sr/90Y (empleando la tecnología de extractores por centrifugado con ácido di-2-ethylhexyl phosphoric D2EHPA) y se compara con otros productos similares. Se describe y propone para el control de la calidad un método estándar para determinar la eficiencia en el marcaje del precursor del 90YCI3 basado en su reacción con DOTA-Tyr3-ostreotido (DOTA-TOC) y separación cromatográfica ITLC-SG.
Key words: fluorine 18, fluorodeoxyglucose, liquid column chromatography, positron computed tomography, quality control, radiopharmaceuticals, toxicity, autoradiolysis
INTRODUCTION
The internal targeted radionuclide therapy for treatment of various diseases in human oncology has recently reached the age of mature. In February 2002, 90Y-ibritumomab tiuxetan (Zevalin; IDEC Pharmaceuticals Corp., San Diego, CA) monoclonal antibody (mAb) received the final approval by the Food and Drug Administration (FDA) as the first commercially available radiolabelled antibody for cancer treatment. Schering received the European approval for Zevalin on January 22, 2004. Zevalin proved high efficiency in treatment of relapsed or refractory non-Hodgkin‘s Lymphoma of B-cells in clinical trials [1]. An overall response rate of 80% and a complete response of 30% were observed compared to the 56% and 16% response for immunnotherapy with Rituximab, unlabelled mAb alone. Since that time, very intensive research of various biomolecules labelled with different therapeutic radionuclides, targeted against the most significant tumour antigens and receptors, has started. For diagnostics of chosen types of tumors, radioactive labelled octreotide derivatives are often indicated. Octreotide is a synthetic somatostatine analogue with a longer biological half-life. It is metabolised in the liver. Much like somatostatine, octreotide binds to the SRIF 1-class SSRT (SRIF: somatotropine release inhibiting factor). 90Y-DOTA-d-Phe1-Tyr3-octreotide (90Y-DOTATOC) has recently been used for treatment of patients with somatostatin receptor positive tumors. A pilot study with 44 patients with advanced somatostatin receptor positive tumors showed that only 29 of the 44 patients could be treated with 90Y-DOTATOC. Taking into account the radiotoxicity of 90Y, a reliable diagnostic tool for examination of the 90Y-DOTATOC affinity to the somatostatin receptor positive tumor is essential to avoid the ¨try and error principal¨ therapy. Patients with a cumulative radiation dose Ð 7400 MBq/m2 showed no severe renal or aematological toxicity, while the patients having received a cumulative radiation dose > 7400 MBq/m2 developed renal and/or haematological toxicity [2]. Therefore, to develop and optimize a new methodology for synthesis of 86/90Y-DOTATOC derivatives appears to be very useful. The biochemical and biophysiological properties of the octreotide derivatives are not influenced by the used yttrium isotope.
Very high demands are imposed on purity of radionuclide precursors used for labelling of biomolecules in nuclear medicine. There are only few b--emitting radionuclides commercially produced in corresponding quality. Among them, no carrier added 90YCl3 radionuclide precursor in diluted 0.04 – 0.05 mol’.l-1 hydrochloric acid is currently the most important because of the very high 90Y specific radioactivity (theoretically about 500 Ci’.mg -1) being reached in the production and the 90Y decay properties (pure b--emission; average energy 0.935 MeV, maximum 2.284 MeV; penetration in tissue 5-9 mm; physical half-life 64.1 h).
Some of the commercially produced and marketed 90Y radionuclide preparations, including their main properties as stated in producer‘s specifications, are presented in table 1.
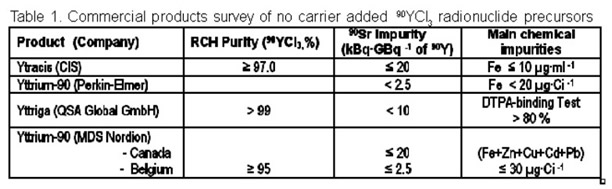
The quality of the latest 90YCl3 commercial product approved in Europe as radiopharmaceutical precursor (Yttriga, AEA Technology, QSA Global GmbH, Braunschweig, Germany) is estimated using the binding efficiency test with DTPA instead of chemical impurities definition. This test seems to be much more predicative and crucial for quality control of preparations than estimating the admissible limits of individual chemical impurities. Determination of all trace impurities and estimation of their acceptable concentrations are nearly impossible. Therefore, a Standard Labelling Test (SLT) using DOTATOC, one of the DOTA conjugated peptides frequently used in RIT preclinical and clinical experiments, has been developed in our laboratory. The results are presented in this contribution.
Experimental
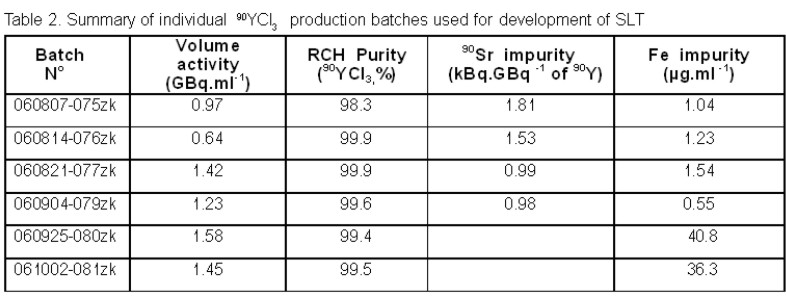
High pure 90YCl3 radionuclide precursor was prepared in our laboratory using the experimental pilot facility with two-stage Centrifugal Semicounterflow Extractors [3]. The separation of 90Y from the 90Sr/90Y mixture in 0.5 mol’.l -1 nitric acid was accomplished by extracting it with di-2-ethylhexylphosphoric acid in n-dodecane. The extraction was followed by washing steps (e.g. with n-hexane) and reextraction of 90Y into 5 mol.l -1 hydrochloric acid. Vacuum evaporation was used for the final concentration. Subsequently, the product was dissolved in a small volume of 0.05 mol.l -1 HCl. The charcteristics of individual production batches used in development of Standard Labelling Test are summarized in table 2. The two last batches significantly contamined by Fe were used to determine the level of Fe resulting in rapid decrease of 90Y labelling efficiency.
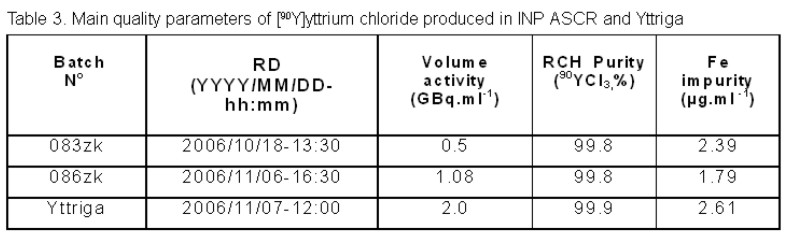
The main quality parameters of our preparation in comparison to the Yttriga product (AEA Technology, QSA Global GmbH, Braunschweig, Germany) are presented in table 3.
DOTA-Tyr3-Octreotide, DOTA-TOC (MW 1421.64 g.mol-1), was supplied by piCHEM, Austria (white lyophilisate, purity > 95% by HPLC).
Labelling Protocol for analytical use [4] : About 2.5–5 MBq of 90YCl3 in 300 µl of 0.05 mol.l-1 HCl was mixed with 5-20 µg of DOTA-TOC in 300 µl of 0.4 mol.l-1 sodium acetate (NaAc – supra pure) and heated in water bath for 5-40 min at 60-95°C in order to find the optimal labelling conditions.
Chromatographic method for determination of 90Y fraction incorporated in DOTA-TOC complex: About 2 µl of the solution after termination of Labelling Protocol was applied on the start (about 10 mm at the margin) of ITLC chromatographic strips (15 x 200 mm, ITLC-SG, Pall Life Sciences, PALL Corporation). Subsequently, the strips were ascendently developed in 0.1 mol.l-1 sodium citrate (Na3Cit) up to about 10 mm bellow the strip margin. The strips were analyzed on 90Y distribution using b--scanner after being air dried. Each sample was analyzed in 3 parallels.
Instruments: Linear scanning of radiochromatograms for 90Y distribution was done by TLC Scanner RAYTEST, Minigita with Proportional Gas Flow (Argon 90%-Methane 10%) Beta Counting Tube (Isotopenmeßgeräte GmbH).
RESULTS AND DISCUSSION
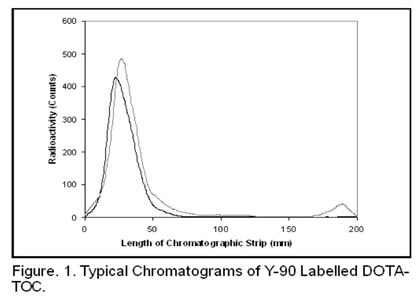
Bifunctional chelators such as 1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-tetraacetic acid (DOTA) and diethylenetriaminepenta -acetic acid (DTPA) [5] are widely used to bound radiometals, namely threevalent lanthanides and actinides, onto biomolecules. The DOTA bioconjugates demonstrate much higher in vivo stability than the DTPA analogues. However, the kinetics of their synthesis (e.g. chelatation of 90Y by DOTA) is significantly slower and requires temperature range up to 100ºC. Nevertheless, they are preferred for use in nuclear medicine, and, thereby, they represent suitable examples for developing SLT. Such test of labelling efficiency reflects credibly the labelling power of 90YCl3 radionuclide precursor in majority of its applications. Very high attainable labelling efficiency despite the presence of chemical impurities (e.g. metal interferents like iron) in low concentrations is an added reason for choosing the 90Y-DOTATOC system for SLT. Figure. 1 demonstrates the typical chromatogram appearance of SLT (Batch No. 060904-079zk of our production - table 2).
Separation of 90Y-DOTATOC complex from free yttrium-90 is quantitative in the presented chromatographic system. These only two components appear in solutions after the labelling procedure described above so that analytical evaluation of results is very easy. More precise determination of optimal labelling conditions (labelling kinetics, incubation temperature, concentration of DOTATOC) is ilustrated in figures 2-4. Obviously, the optimal incubation temperature (maximum yield) is 80°C. Higher temperature may cause degradation of the peptide resulting in decreased labelling yields. The optimal reaction time seems to be 25-30 minutes because of slower formation kinetics of DOTA complexes.
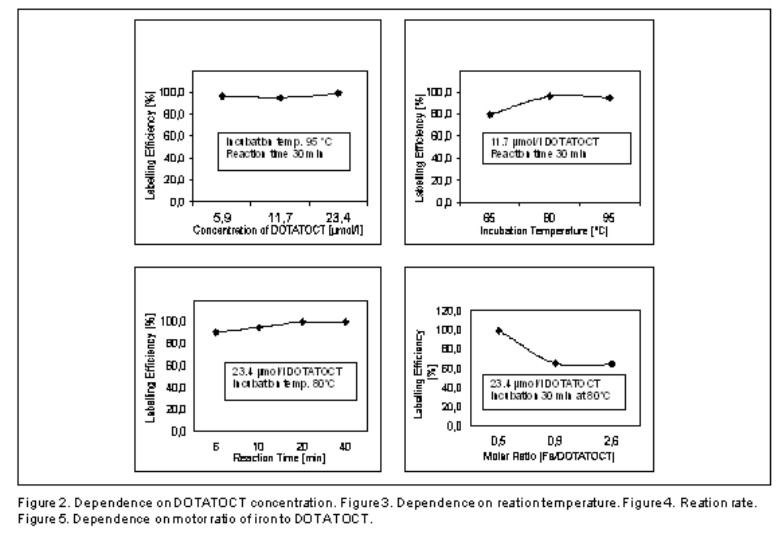
The Standard Labellig Protocol was precised as follows:
About 2.5–5 MBq of 90YCl3 in 300 µl of 0.05 mol.dl-1 HCl is mixed with 20 µg of DOTATOCT in 300 µl of 0.4 mol.l-1 sodium acetate (final concentration of DOTATOCT 23.4 µmol/l) and heated in water bath for 30 min at 80°C. ITLC chromatographic procedure (see page 4) is then applied (after cooling down the sample to the room temperature) to determine 90YCl3 labelling efficiency. Each chromatogram is developed in 3 parallels. Two production batches of 90YCl3 radionuclide recursor with higher Fe content (080zk and 081zk – see table 2) were analyzed using this method (SLT) in order to determine maximum permissible limit for Fe impurity. This limit corresponds to toichiometric saturation of DOTATOCT (figure 5). Acceptable labelling yield was obtained up to molar ratio [Fe/DOTATOCT] = 0.5.
The SLT developed in our laboratory and described above was used to estimate the labelling efficiency of two 90YCl3 radionuclide precursor products compiled in table 3: Yttriga-06/11/07, QSA Global GmbH and our product Batch No. 086zk-06/11/06. Labelling efficiency (mean ± SD, n=3) for both products was: 99.5 ± 0.1% for Yttriga and 96.1 ± 0.8% for our product.
REFERENCES
[1] WITZIG, T. E., GORDON, L. I., CABANILLAS, F., CZUCZMAN, M. S., EMMANOUILIDES, Ch. JOYCE, R., POHLMAN, B. L. BARTLETT, N. L. WISEMAN, G.A., PADRE, N. A. GRILLO-LÓPEZ, MULTANI, J. P., WHITE, Ch.A., Randomized Controlled Trial of Yttrium-90–Labeled Ibritumomab Tiuxetan Radioimmunotherapy Versus Rituximab Immunotherapy for Patients With Relapsed or Refractory Low-Grade, Follicular, or Transformed B-Cell Non-Hodgkin’s Lymphoma, Journal of Clinical Oncology, Vol 20, Issue 10 (May) 2453-2463(2002).
[2] OTTE, A., HERRMANN, R., HEPPELER, A., BEHE,M., JERMANN, E. POWELL, P. MAECKE, H.R., MÜLLER,J. , Yttrium-90-DOTATOC: first clinical results. Eur J Nucl Med, Vol. 26 (1999)1439-1447.
[3] KODINA, G. E., G. KORPUSOV, V., FILYANIN, A. T., Production of High-Purity 90Y on
Specially Developed Centrifugal Semicounterflow Extractors, Radiochemistry, Vol. 44, No. 1, (2002)62-66.
[4] RÖSCH, F., 86/90Y-DOTA-octreotide Labelling Protocol, Personal Communication, 2006, University Mainz, Institute of Nuclear Chemistry.
[5] BREEMAN, W.A., JONG, M. De., VISSER,T,J., ERION, J.L., KRENNING, E.P., Optimizing Conditions for Radiolabeling of DOTA-Peptides with 90Y, 111I and 177 Lu at High Specific Activities, European Journal of Nuclear Medicine Molecular Imaging, Vol. 30 (2003)917-920.
Recibido: 9 de enero de 2007
Aceptado: 1 de noviembre de 2007













