Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Nucleus
versión impresa ISSN 0864-084X
Nucleus no.52 Ciudad de La Habana jul.-dic. 2012
CIENCIAS NUCLEARES
Is Technetium-99m dead or still alive? An outlook to recent developments with specialfocus on myocardial perfusion imaging
¿Murió o permanece con vida el Tecnecio 99m? Una panorámica de desarrollos recientes con énfasis en la imagen de perfusión del miocardio
Adriano Duatti
Laboratory of Nuclear Medicine, Department of Radiological Sciences, University of Ferrara, 44121 Ferrara, Italy, Industrial Application and Chemistry Section, Division of Physical and Chemical Sciences, Department of Nuclear Sciences and Applications, International Atomic Energy Agency, 1400 Vienna, Austria
ABSTRACT
Despite the advances associated with the introduction of PET technology, reasons to consider that SPECT, particularly with ![]() radiopharmaceuticals will continue playing an important role in Nuclear Medicine, are presented. The details examined are the following ones. An improvement of the technology SPECT is appreciated with the development of new detection systems and the advantages of the appearance of the hybrid systems SPECT / TAC. The biggest half-life of the main SPECT radionuclides like
radiopharmaceuticals will continue playing an important role in Nuclear Medicine, are presented. The details examined are the following ones. An improvement of the technology SPECT is appreciated with the development of new detection systems and the advantages of the appearance of the hybrid systems SPECT / TAC. The biggest half-life of the main SPECT radionuclides like ![]() and
and ![]() in comparison with PET radionuclides, facilitates its transportation at larger distances, allow the realization of studies with radiopharmaceuticals with different radionuclides in the same patient, and the possibility of the detection of lesions of low tracer accumulation. Finally the main base core for
in comparison with PET radionuclides, facilitates its transportation at larger distances, allow the realization of studies with radiopharmaceuticals with different radionuclides in the same patient, and the possibility of the detection of lesions of low tracer accumulation. Finally the main base core for ![]() complex formation with different ligands are reviewed, as a background that assure the appearance of new radiopharmaceuticals of practical interest. The oncological and cardiological potential applications are examined with emphasis in these last ones. It is clear that
complex formation with different ligands are reviewed, as a background that assure the appearance of new radiopharmaceuticals of practical interest. The oncological and cardiological potential applications are examined with emphasis in these last ones. It is clear that ![]() radiopharmacy will continue playing an important role in nuclear medicine.
radiopharmacy will continue playing an important role in nuclear medicine.
Key words: myocardium, Technetium 99, perfused organs, radiopharmaceuticals, nuclear medicine, single photon emission computed tomography.
RESUMEN
En el trabajo se exponen razones para considerar que el SPECT, particularmente con radiofármacos de ![]() continuará teniendo un importante papel en medicina nuclear, no obstante los avances asociados a la incorporación de la tecnología PET. Las razones que se examinan son las siguientes. Se aprecia un mejoramiento de la tecnología SPECT con el desarrollo de nuevos sistemas de detección y las ventajas de la aparición de los sistemas híbridos SPECT/TAC, la mayor vida media de los principales radionúclidos SPECT como
continuará teniendo un importante papel en medicina nuclear, no obstante los avances asociados a la incorporación de la tecnología PET. Las razones que se examinan son las siguientes. Se aprecia un mejoramiento de la tecnología SPECT con el desarrollo de nuevos sistemas de detección y las ventajas de la aparición de los sistemas híbridos SPECT/TAC, la mayor vida media de los principales radionúclidos SPECT como ![]() y
y ![]() en comparación con los radionúclidos PET, lo que posibilita su traslado a mayores distancias, la realización de estudios con radiofármacos con más de un radionúclido en el mismo paciente y la posibilidad de detectar lesiones de baja captación, favorecido por lo indicado de las vidas medias. Finalmente se examinan los principales núcleos base de formación de complejos de
en comparación con los radionúclidos PET, lo que posibilita su traslado a mayores distancias, la realización de estudios con radiofármacos con más de un radionúclido en el mismo paciente y la posibilidad de detectar lesiones de baja captación, favorecido por lo indicado de las vidas medias. Finalmente se examinan los principales núcleos base de formación de complejos de ![]() con distintos ligandos que aseguran la aparición de nuevos radiofármacos de interés. Se examinan las potenciales aplicaciones oncológicas y cardiológicas con énfasis en estas últimas. Se considera que la radiofarmacia del
con distintos ligandos que aseguran la aparición de nuevos radiofármacos de interés. Se examinan las potenciales aplicaciones oncológicas y cardiológicas con énfasis en estas últimas. Se considera que la radiofarmacia del ![]() continuará jugando un importante papel en medicina nuclear.
continuará jugando un importante papel en medicina nuclear.
Palabras claves: miocardio, Tecnecio 99, órganos perfundidos, radiofármacos, medicina nuclear, tomografía de emisión computarizada de fotón único.
INTRODUCTION
The radionuclide Technetium-99m has been the workhorse of nuclear medicine for decades. Nuclear imaging would not have been existed without this wonderful radionuclide having almost ideal nuclear properties for yielding functional images of the internal organs of the body. After so many successful years, as in any fairy tales, now it seems that time has come for it to disappear from the scene almost completely replaced by positron-emitting radionuclides. Is this really the end of the story? We will try here to bring some arguments that refute this conclusion and support the viewthat ![]() radiopharmaceuticals may still have an important role in diagnostic nuclear medicine and molecular imaging. This short review aims to provide a few examples demonstrating how the field is still active and producing new potentially useful radiopharmaceuticals selectively visualizing exactly the same biological end points that are currently the most widely pursuedusing positron-emitting tracers.
radiopharmaceuticals may still have an important role in diagnostic nuclear medicine and molecular imaging. This short review aims to provide a few examples demonstrating how the field is still active and producing new potentially useful radiopharmaceuticals selectively visualizing exactly the same biological end points that are currently the most widely pursuedusing positron-emitting tracers.
Some preliminary considerations
The nuclear imaging technologies, PET and SPECT are highly analogous. They both offer the sensitivity required to monitor drug distribution, pharmacokinetics and pharmacodynamics, and for imaging specific biomarkers and molecular end points. Depending on the ligands and radionuclides used, a myriad of molecular targets can be hit.
Traditionally, PET has outperformed SPECT in terms of detection sensitivity and image resolution. Positron emitters have higher tissue penetration than the single photon emitters and the ability to localize positrons without the use of collimation techniques, results in better detection sensitivities. However, the recent emergence of hybrid systems, PET/CT and SPECT/CT, has narrowed some of the differences between the two modalities. Indeed, CT image data can be used to correct for tissue attenuation, and this anatomical information further contributes to improve the localization of single-photon emissions. Furthermore, the recent introduction of solid-state detectors and photomultipliers has dramatically improved the performance of SPECT.
Currently, the impact of these technological advancements has become apparent particularly in the field of imaging of smaller subjects such as mice and other animals. In fact, the recent invention of multiple pinhole collimation systems has resulted in very high imaging sensitivities with spatial image resolution in the nanoliter range, far superior to PET.
Traditional single photon emitting radioisotopes such a ![]() and
and ![]() have radioactive half-lives ofhours and are long enough to allow their production at a central site for subsequent distribution over a relatively large geographic region. In contrast most PET isotopes decay much faster and the distribution of their radiopharmaceuticals is impossible or usually requires building up a complex and expensive infrastructure. Conversely, SPECT is faster and cheaper when there is already a registered radiopharmaceutical for a specific diagnostic indication.
have radioactive half-lives ofhours and are long enough to allow their production at a central site for subsequent distribution over a relatively large geographic region. In contrast most PET isotopes decay much faster and the distribution of their radiopharmaceuticals is impossible or usually requires building up a complex and expensive infrastructure. Conversely, SPECT is faster and cheaper when there is already a registered radiopharmaceutical for a specific diagnostic indication.
The ultra-high resolution nuclear imaging capabilities of novel SPECT/CT scanners offer unique advantages when the concentration of the target is relatively low. In these situations, detecting a signal sometimes requires a very low level of background noise that can be reachedafter hours necessary to eliminate background activity. For these applications, single photon emitters with longer half-lives can be more effective than short-lived positron emitting isotopes.
Technetium 99m cores
The most interesting Tc-99m radiopharmaceuticals that have been developed in the last decade belong almost exclusively to three main categories of complexes each characterized by a specific metallic core. These cores constitute characteristic chemical motifs that control and shape the molecular structure of the resulting radiopharmaceutical and, ultimately, its biological properties. The main features of these cores and of the corresponding complexes are briefly outlined in the following.
A terminal ![]() triple bond can be formally viewed as generated by the bonding interaction between a
triple bond can be formally viewed as generated by the bonding interaction between a ![]() ion and a
ion and a ![]() nitrido nitrogen atom. This yields the
nitrido nitrogen atom. This yields the ![]() functional moiety that, owing to its small molecular size, behave like a single atomic entity. Nitrido Tc-99m complexes usually possess a five-coordinated geometry in which the terminal
functional moiety that, owing to its small molecular size, behave like a single atomic entity. Nitrido Tc-99m complexes usually possess a five-coordinated geometry in which the terminal ![]() groupspans an apical position. These compoundsare commonly classified in two distinct subtypes: (a) symmetrical and (b) asymmetrical complexes [1-3].
groupspans an apical position. These compoundsare commonly classified in two distinct subtypes: (a) symmetrical and (b) asymmetrical complexes [1-3].
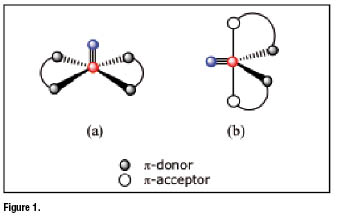
Symmetrical complexes formwhen two identical bidentate chelating ligands bind the same ![]() group (figure 1). This class can be further separated in two subsets depending on which molecular arrangement,among the two allowed for a five-coordinated species, is preferred by the symmetrical complex. Five-coordinated complexes can take on either a square pyramidal (sp) or a trigonal bipyramidal (tbp) arrangement Experimental evidence showed that square pyramidal complexes (figure 1a) are exclusively formed when two bidentate ligands having
group (figure 1). This class can be further separated in two subsets depending on which molecular arrangement,among the two allowed for a five-coordinated species, is preferred by the symmetrical complex. Five-coordinated complexes can take on either a square pyramidal (sp) or a trigonal bipyramidal (tbp) arrangement Experimental evidence showed that square pyramidal complexes (figure 1a) are exclusively formed when two bidentate ligands having ![]() -donor atoms are utilizedas coordinating sites. In these complexes, the four
-donor atoms are utilizedas coordinating sites. In these complexes, the four ![]() -donors are positioned on the basal plane of the square pyramid, the
-donors are positioned on the basal plane of the square pyramid, the ![]() moiety lying above this plane. Conversely, formation of tbp complexes (figure 1b) usually occurs when a combination of two
moiety lying above this plane. Conversely, formation of tbp complexes (figure 1b) usually occurs when a combination of two ![]() -donor and two
-donor and two ![]() -acceptor atoms is placed around the
-acceptor atoms is placed around the ![]() group. Obviously, symmetrical complexes can be obtained only when each bidentate ligand separately carries a set of one
group. Obviously, symmetrical complexes can be obtained only when each bidentate ligand separately carries a set of one ![]() -donor and one
-donor and one ![]() -acceptor coordinating atoms. As a general rule, the two
-acceptor coordinating atoms. As a general rule, the two ![]() -acceptor atoms on the two bidentate ligands have the tendency to occupy the two axial positions of the tbp geometry, whereas the two
-acceptor atoms on the two bidentate ligands have the tendency to occupy the two axial positions of the tbp geometry, whereas the two ![]() -donors span the positions on the equatorial plane along with the nitrido nitrogen atom of the
-donors span the positions on the equatorial plane along with the nitrido nitrogen atom of the ![]() group.
group.
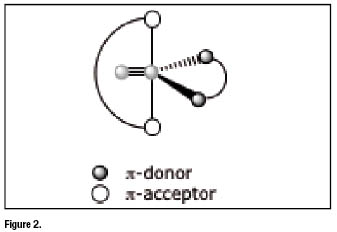
Asymmetrical nitrido complexes [4-8] arise when the two ![]() -acceptor and the two
-acceptor and the two ![]() -donor atoms are separated in two distinct sets belongingtotwo different bidentate ligands. In this situation, upon coordination, the
-donor atoms are separated in two distinct sets belongingtotwo different bidentate ligands. In this situation, upon coordination, the ![]() core becomes surrounded by two bidentate ligands that are not identical since one carries only
core becomes surrounded by two bidentate ligands that are not identical since one carries only ![]() -acceptor atoms and the other only
-acceptor atoms and the other only ![]() -donor atoms. Accordingly, the final nitride complex (heterocomplex) has an asymmetrical structure as illustrated figure 2 below.
-donor atoms. Accordingly, the final nitride complex (heterocomplex) has an asymmetrical structure as illustrated figure 2 below.
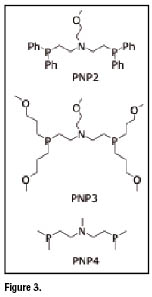
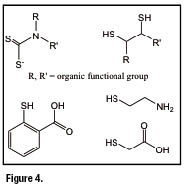
Common bidentate ![]() -acceptor ligands utilized in the preparation of asymmetrical complexes are heterodiphosphane ligands of the type shown in figure 3 (PNP ligands). Examples of bidentate ligands having
-acceptor ligands utilized in the preparation of asymmetrical complexes are heterodiphosphane ligands of the type shown in figure 3 (PNP ligands). Examples of bidentate ligands having ![]() , S,
, S, ![]() , and
, and ![]() as
as ![]() -donor atoms (XY ligands) are illustrated in figure 4. Asymmetrical nitrido heterocomplexes, therefore, can be represented by the general formula
-donor atoms (XY ligands) are illustrated in figure 4. Asymmetrical nitrido heterocomplexes, therefore, can be represented by the general formula ![]() where the neutral or monopositive charge depends on whether monoanionic or dianionic bidentate XY ligandsare employed in the preparation.
where the neutral or monopositive charge depends on whether monoanionic or dianionic bidentate XY ligandsare employed in the preparation.
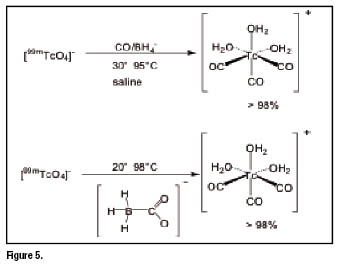
Another important Tc-99m core is the monocationic organometallic fragment ![]() . It represents a building block particularly suitable for the labelling of biomolecules since it contains three fixed CO ligands bound to a formally
. It represents a building block particularly suitable for the labelling of biomolecules since it contains three fixed CO ligands bound to a formally ![]() ion, that constitutes the invariant and stable region of the resulting radiopharmaceuticals. The metallic fragment is completed by the coordination of three weakly bound water molecules, which can be easily exchanged by other chelators for labelling of targeting biomolecules or for the preparation of technetium essential radiopharmaceuticals. The preparation of the intermediated precursor
ion, that constitutes the invariant and stable region of the resulting radiopharmaceuticals. The metallic fragment is completed by the coordination of three weakly bound water molecules, which can be easily exchanged by other chelators for labelling of targeting biomolecules or for the preparation of technetium essential radiopharmaceuticals. The preparation of the intermediated precursor ![]() is not straightforward, but it can be prepared through a kit formulation that is currently available commercially [9-11]. The approach is of broad applicability and many biomolecules have been labelled with the
is not straightforward, but it can be prepared through a kit formulation that is currently available commercially [9-11]. The approach is of broad applicability and many biomolecules have been labelled with the ![]() core. Among these are peptides [12-19], antibodies [18], glucose, CNS receptor ligands and many small molecules [20-24]. The principle of the precursor preparation is given in figure 5 below.
core. Among these are peptides [12-19], antibodies [18], glucose, CNS receptor ligands and many small molecules [20-24]. The principle of the precursor preparation is given in figure 5 below.
The advantages and disadvantages of the ![]() labelling approach have been discussed in more detail in a controversial article [25,26]. A major advantage is certainly the very wide variety of ligands, which bind very efficiently to the Tc(I) metallic center. Complex stabilities are governed by kinetic stability or inertness. The complexes are all highly robust and, commonly, do not decompose in serum or in vivo. Many different ligands have been exploited and among them are hydrides [27], and cyclopentadienyl [28]. Many combinations of mono- bi- and tridentate ligands are possible, but tridentate chelators turned out to be most versatile [29]. Labelling can be performed at low biomolecule concentration, but heating is always required in order to achieve quantitative labelling. The resulting complexes are usually lipophilic, which could be a disadvantage when attempting to labelhydrophilic substrates such as peptides. Despite of this, a few works described the preparation of very hydrophilic Tc-99m tris-carbonyl complexes [19].
labelling approach have been discussed in more detail in a controversial article [25,26]. A major advantage is certainly the very wide variety of ligands, which bind very efficiently to the Tc(I) metallic center. Complex stabilities are governed by kinetic stability or inertness. The complexes are all highly robust and, commonly, do not decompose in serum or in vivo. Many different ligands have been exploited and among them are hydrides [27], and cyclopentadienyl [28]. Many combinations of mono- bi- and tridentate ligands are possible, but tridentate chelators turned out to be most versatile [29]. Labelling can be performed at low biomolecule concentration, but heating is always required in order to achieve quantitative labelling. The resulting complexes are usually lipophilic, which could be a disadvantage when attempting to labelhydrophilic substrates such as peptides. Despite of this, a few works described the preparation of very hydrophilic Tc-99m tris-carbonyl complexes [19].
Heart Imaging
Myocardial perfusion imaging (MPI) still constitutes a major area of diagnostic nuclear medicine where Tc-99m cardiac tracers play a fundamental role. Actually, MPI using Tc-99m radiopharmaceuticals remains the most common, non-invasive imaging tool for risk stratification of patients with coronary artery disease (CAD).
Recent progress in SPECT technology with the introduction of ultrafast dedicated cardiac scanner paralleled the continuous development of new Tc-99m heart imaging agents having biodistribution properties approaching the requirements for an ideal perfusion tracer. Ultra-fast cardiac SPECT cameras have been created to meet current evolutionary challenges in nuclear cardiology [7,8]. These new devices feature high sensitivity as well as improved spatial, temporal and energy resolution. They enable reduction of acquisition time and fast protocols. Most importantly, they are inherently tomographic imaging characterized by high count rate linearity and, therefore,are potentially capable of dynamic 3-D acquisition. In the near future, increased resolution will be a key factor to allow nuclear cardiology entering the field of molecular imaging. Unlike PET, SPECT is capable of simultaneous multiple isotope imaging and, in principle, a better spatial resolution may ultimately be achieved with SPECT. Critical research targets include imaging of vulnerable plaques at high risk for rupture, left ventricular remodelling, angiogenesis, apoptosis and hypoxia, gene expression, and stem cell therapy. Thus a steady evolution of nuclear cardiology beyond the assessment of myocardial perfusion, towards the characterization of molecular events can be envisaged, thus linking molecular biology science and clinical cardiology.
In the following, a brief overview of recent progress in the discovery of new Tc-99m cardiac agents using the nitrido and tris-carbonyl Tc-99m cores is reported.
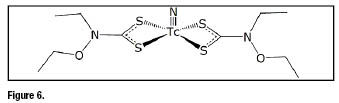
Symmetrical nitrido Tc-99m complexes have proven to be particularly useful for heart imaging the most important class of cardiac tracers being that of bis(dithiocarbamato) nitrido Tc-99m complexes formed by complexes containing two dithiocarbamate ligands bound to a ![]() core [30]. These complexes are neutral and accumulate in myocardium of various animal species and of humans to a different extent depending on the nature of the lateral groups appended to the nitrogen atom of the dithiocarbamate ligand. Extensive clinical studies have been carried out with the complex bis[(N-ethoxy, N-ethyl)dithiocarbamato] nitrido Tc-99m (
core [30]. These complexes are neutral and accumulate in myocardium of various animal species and of humans to a different extent depending on the nature of the lateral groups appended to the nitrogen atom of the dithiocarbamate ligand. Extensive clinical studies have been carried out with the complex bis[(N-ethoxy, N-ethyl)dithiocarbamato] nitrido Tc-99m (![]() N-NOET) (figure 6) [30,31].
N-NOET) (figure 6) [30,31]. ![]() N-NOET) [32-46] was found to accumulate human myocardium with uptake as high as 4% of the injected activity at 5 min post injection.
N-NOET) [32-46] was found to accumulate human myocardium with uptake as high as 4% of the injected activity at 5 min post injection.
This compound showed high first-pass extraction (> 86%) in a canine and isolated rabbit heart models, and a myocardial uptake that correlates with myocardial blood flow over a wide range of flow. According to the observed kinetic behavior, ![]() -N-NOET appears much closer to
-N-NOET appears much closer to ![]() than other technetium complexes. These similarities include high first-pass extraction, good correlation with coronary blood flow, and redistribution. In fact, as observed for
than other technetium complexes. These similarities include high first-pass extraction, good correlation with coronary blood flow, and redistribution. In fact, as observed for ![]() ,experimental studies showed that
,experimental studies showed that ![]() -N-NOET has differential clearance from ischemic and normal myocardium resulting in normalization of initial ischemic defect over time. Models of occlusion and reperfusion demonstrate significant redistribution of
-N-NOET has differential clearance from ischemic and normal myocardium resulting in normalization of initial ischemic defect over time. Models of occlusion and reperfusion demonstrate significant redistribution of ![]() N-NOET within 15 minutes after injection similar to that observed for the neutral lipophilic compound
N-NOET within 15 minutes after injection similar to that observed for the neutral lipophilic compound ![]() -Teboroxime.
-Teboroxime.
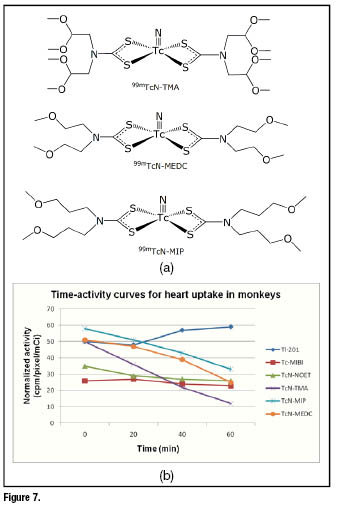
A relevant feature of symmetrical bis(dithiocarbamato) ![]() nitrido tracers lies in the possibility toachieve a fine tuning of their pharmacokinetics properties by simply modifying the chemical nature of the lateral groups bound to the dithiocarbamate [>N-C(=S)
nitrido tracers lies in the possibility toachieve a fine tuning of their pharmacokinetics properties by simply modifying the chemical nature of the lateral groups bound to the dithiocarbamate [>N-C(=S)![]() ] moiety. This allowed obtaining a series of perfusion tracers showing a variable residence time in myocardial tissue ranging from a few minutes to hours. For instance, the three derivatives represented in figure 7a only differ in their lateral groups, but their heart washouts are dramatically different. In figure 7b, the time-variation of heart uptake in monkeys for these compounds isreported in comparison with
] moiety. This allowed obtaining a series of perfusion tracers showing a variable residence time in myocardial tissue ranging from a few minutes to hours. For instance, the three derivatives represented in figure 7a only differ in their lateral groups, but their heart washouts are dramatically different. In figure 7b, the time-variation of heart uptake in monkeys for these compounds isreported in comparison with ![]() ,
, ![]() -MIBI and
-MIBI and ![]() N-NOET.
N-NOET.
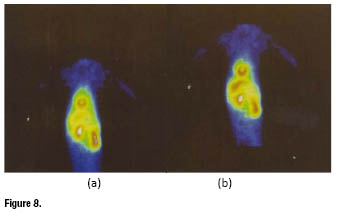
The first-pass extractionsof these tracers were comparable to that of ![]() and
and ![]() N-NOET, as well as the high initial cardiac accumulation. However, after 20 min post injection, approximately 50% of the initial activity was washed out. A representative example of planar images recorded in baboons at different times [5 min (a) and 25 min (b)] for the complex
N-NOET, as well as the high initial cardiac accumulation. However, after 20 min post injection, approximately 50% of the initial activity was washed out. A representative example of planar images recorded in baboons at different times [5 min (a) and 25 min (b)] for the complex ![]() -TMIP is reported in figure 8.
-TMIP is reported in figure 8.
The high first-pass extraction combined with a fairly linear relationship between uptake and flow suggest that this class of myocardial tracers could be particularly useful when used in combination with the new dedicated ultrafast cardiac scanners able to collect high-quality SPECT images in less than 2 minutes.
Replacement of one dithiocarbamato ligand with an heterodiphosphane ligand PN(R)P led to the formation of asymmetrical species containing two different bidentate ligands bound to the same ![]() group. This change yielded dramatic consequences on the observed cardiac uptake. The resulting monocationic nitrido Tc(V) heterocomplexes,
group. This change yielded dramatic consequences on the observed cardiac uptake. The resulting monocationic nitrido Tc(V) heterocomplexes, ![]() ,constitute a novel class of myocardial tracers exhibiting superior imaging qualities was obtained [11, 41]. In particular, using the heterodiphosphane ligand bis[(dimethoxypropylphosphanyl)ethyl]ethoxyethylamine (PNP5) a novel class of myocardial tracers exhibiting superior imaging qualities was obtained [47,48]. Within this class, the derivative
,constitute a novel class of myocardial tracers exhibiting superior imaging qualities was obtained [11, 41]. In particular, using the heterodiphosphane ligand bis[(dimethoxypropylphosphanyl)ethyl]ethoxyethylamine (PNP5) a novel class of myocardial tracers exhibiting superior imaging qualities was obtained [47,48]. Within this class, the derivative![]() (
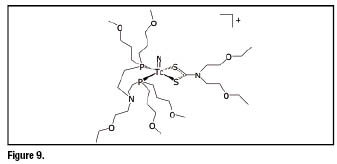
(![]() N-DBODC) (DBODC = diethoxyethyldithiocarbamato, PNP5 = bis[(dimethoxypropylphosphanyl)ethyl]ethoxyethylamine) has recently raised much interest due to its unprecedented imaging properties. The structure of this complex is reported in figure 9.
N-DBODC) (DBODC = diethoxyethyldithiocarbamato, PNP5 = bis[(dimethoxypropylphosphanyl)ethyl]ethoxyethylamine) has recently raised much interest due to its unprecedented imaging properties. The structure of this complex is reported in figure 9.
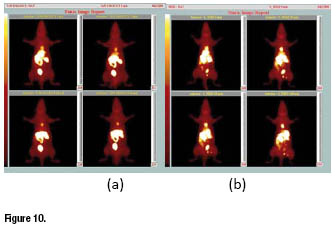
In figure 10a, whole-body image of a rat obtained at 30 min after administration of the tracer is reported. It clearly shows a high accumulation of activity in the myocardium, but with negligible uptake in background tissues, particularly in lungs and liver. As a result, heart region appears very well delineated allowing the acquisition of SPECT images of superior quality. The difference in the liver washout kinetic between the new tracer and ![]() -Sestamibi is clearly evidenced in figure 10b, which reports rat planar whole-body image for
-Sestamibi is clearly evidenced in figure 10b, which reports rat planar whole-body image for ![]() -Sestamibi collectedat the same time.
-Sestamibi collectedat the same time.
Biodistribution data of ![]() N-DBODC in rats [49−51] showed that the heart is the main target organ and liver activity is rapidly washed out into the intestine leaving the cardiac region completely free of background activity. This result appeared particularly evident when time variation of heart/liver ratiois considered in comparison with
N-DBODC in rats [49−51] showed that the heart is the main target organ and liver activity is rapidly washed out into the intestine leaving the cardiac region completely free of background activity. This result appeared particularly evident when time variation of heart/liver ratiois considered in comparison with ![]() -sestamibi and
-sestamibi and ![]() -tetrofosmin (figure 11). Heart/liver ratio of
-tetrofosmin (figure 11). Heart/liver ratio of ![]() N-DBODC rises dramatically over time whereas those of the other two agents remain almost constant.
N-DBODC rises dramatically over time whereas those of the other two agents remain almost constant.
First-pass extraction fraction of ![]() N-DBODC, as determined in dogs [52], was found to be comparable to
N-DBODC, as determined in dogs [52], was found to be comparable to ![]() -sestamibi and
-sestamibi and ![]() -tetrofosmin.The new tracer has been tested in healthy human volunteers and results confirmed the favorable kinetic behavior and fast and quantitative liver washout as early as 30 min post injection [53].
-tetrofosmin.The new tracer has been tested in healthy human volunteers and results confirmed the favorable kinetic behavior and fast and quantitative liver washout as early as 30 min post injection [53].
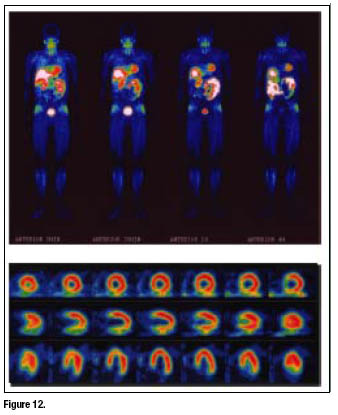
Whole-body images and high-quality SPECT images of the myocardium (figure 12) were collected starting from 5 min after tracer administration. Radioactivity in the liver decreased rapidly whereas a slow washout of myocardial activity was observed. Thus, the combination of the rapid liver clearance and the high cardiac accumulation may shorten the duration of imaging protocols by allowing earlier image acquisition.
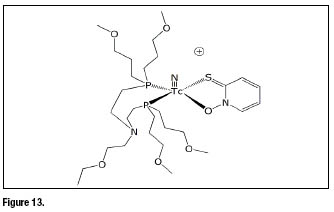
Other bidentate chelators have been used to replace dithiocarbamate ligands in the basic structure of ![]() -DBODC.The ligand 2-mercaptopyridine oxide were employed in the preparation of the asymmetrical compound shown in figure 13. This complex has, essentially, the same basic structure of
-DBODC.The ligand 2-mercaptopyridine oxide were employed in the preparation of the asymmetrical compound shown in figure 13. This complex has, essentially, the same basic structure of ![]() -DBODC, but differs from the bidentate
-DBODC, but differs from the bidentate ![]() -donor chelating ligand.
-donor chelating ligand.
Preliminary biological evaluation of ![]() N-MPO in rats [54, 55] showed a high initial heart uptake with long myocardial retention. The heart uptake of
N-MPO in rats [54, 55] showed a high initial heart uptake with long myocardial retention. The heart uptake of ![]() N-MPO was between that of
N-MPO was between that of ![]() -sestamibi and that of
-sestamibi and that of ![]() N-DBODC. Clearance from the liver and lungs was rapid, resulting in high heart-liver and heart-lung ratios.
N-DBODC. Clearance from the liver and lungs was rapid, resulting in high heart-liver and heart-lung ratios.
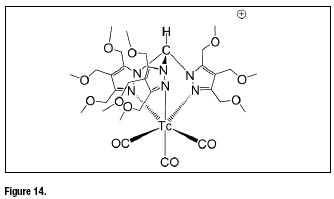
In the family of Tc-99m tris-carbonyl complexes, recently a new promising heart imaging agent has been described [56,57]. The complex fac-![]() containing a functionalized tris(pyrazolyl)methane chelators with methoxymethyl groups at the azole rings (figure 14) exhibits a significantly high and stable heart uptake associated with avery fast liver clearance.
containing a functionalized tris(pyrazolyl)methane chelators with methoxymethyl groups at the azole rings (figure 14) exhibits a significantly high and stable heart uptake associated with avery fast liver clearance.
Cancer imaging
Somatostatin receptors (SSTs) are integral membrane glycoproteins that are distributed in a variety of tissues throughout the body [22]. They have multiple physiological functions, including an inhibition of secretion of a growth hormone, glucagon, insulin, gastrin and other human peptide hormones. In addition, somatostatin plays an inhibitory role in the immune system and acts as a neuromodulatory peptide in the central nervous system. Alterations of somatostatin receptor expression during disease, such as overexpression in many tumours can be exploited by imaging techniques. Receptor-positive tumours may be originated in the neuroendocrine system, such as pituitary adenomas, gastroenteropancreatic tumours, and pancreatic islet-cell tumours, but other tumours, such as lymphomas and breast cancer, may possess these receptors as well. Of the six SST subtypes, SST1, SST2A, SST2B, SST3, SST4, and SST5, SST2A is the most important one because of its high overexpression in malignant tumours and its high affinity for the clinically available somatostatin analogues.
The first successful Tc-99m radiopharmaceutical targeting SST receptors that has beenintroduced into the clinical use was P829 (Depreotide, NeoSPECT) [58]. It is based on the oxo-Tc-99m core coordinated to a ![]() monothiol-bisamide-monoamine ligand bearing an additional lysine residue. In patients, it has proven successful in imaging solitary pulmonary nodes but failed in gastrointestinal tumours. Further improvement of the peptide (
monothiol-bisamide-monoamine ligand bearing an additional lysine residue. In patients, it has proven successful in imaging solitary pulmonary nodes but failed in gastrointestinal tumours. Further improvement of the peptide (![]() -P2045) resulted in a dramatic increase in tumour uptake with improved biodistribution pattern suitable for
-P2045) resulted in a dramatic increase in tumour uptake with improved biodistribution pattern suitable for ![]() labelling for therapeutic applications [59].
labelling for therapeutic applications [59].
HYNIC (6-hydazinonicotinamide) is of particular interest due to its high ![]() -labeling efficiency (rapid and high yield radiolabeling), the high solution stability of its
-labeling efficiency (rapid and high yield radiolabeling), the high solution stability of its ![]() complexes, and the easy use of coligands for modification of biodistribution characteristic of the
complexes, and the easy use of coligands for modification of biodistribution characteristic of the ![]() -labeled small biomolecules [60]. HYNIC-conjugated octreotide analogues have been proven to be particularly suitable for
-labeled small biomolecules [60]. HYNIC-conjugated octreotide analogues have been proven to be particularly suitable for ![]() labelling [61]. Among them,
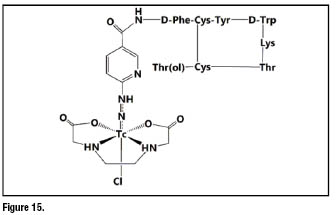
labelling [61]. Among them, ![]() -octreotide (figure 15) was proved to be superior in patients compared to
-octreotide (figure 15) was proved to be superior in patients compared to ![]() -DTPA octreotide. Especially when EDDA (ethylenediaminodiacetic acid) was used as the coligand, the resulting
-DTPA octreotide. Especially when EDDA (ethylenediaminodiacetic acid) was used as the coligand, the resulting ![]() -labeled peptide becomes highly stable and hydrophilic. So far
-labeled peptide becomes highly stable and hydrophilic. So far ![]() -EDDA/HYNIC-TOC can be considered the most widely used
-EDDA/HYNIC-TOC can be considered the most widely used ![]() labelled octreotide derivative [62].
labelled octreotide derivative [62].
Tumors produce many angiogenic factors, which are able to activate endothelial cells in the established blood vessels and induce endothelial proliferation, migration, and new vessel formation (angiogenesis). The angiogenic process is regulated by cell adhesion receptors. Integrins are such a family of proteins that facilitate cellular adhesion and migration on the extracellular matrix proteins found in intercellular spaces and basement membranes, and regulate cellular entry and withdraw from the cell cycle [63]. Integrin ![]() serves as a receptor for the extracellular matrix proteins, including vitronectin, fibronectin, fibrinogen, lamin, collagen, Von Willibrand’s factor, osteoponin and adenovirus particles, with the exposed arginine-glycine-aspartic (RGD) tripeptide sequence [64,65]. Integrin
serves as a receptor for the extracellular matrix proteins, including vitronectin, fibronectin, fibrinogen, lamin, collagen, Von Willibrand’s factor, osteoponin and adenovirus particles, with the exposed arginine-glycine-aspartic (RGD) tripeptide sequence [64,65]. Integrin ![]() is expressed at low levels on epithelial cells and mature endothelial cells, but it is highly expressed on the activated endothelial cells in the neovasculature of tumors. The highly restricted expression of integrin
is expressed at low levels on epithelial cells and mature endothelial cells, but it is highly expressed on the activated endothelial cells in the neovasculature of tumors. The highly restricted expression of integrin ![]() during tumor growth, invasion and metastasis present an interesting molecular target for early diagnosis of rapidly growing and metastatic tumors. During the last several years, a number of radiolabeled RGD peptides have been evaluated as integrin
during tumor growth, invasion and metastasis present an interesting molecular target for early diagnosis of rapidly growing and metastatic tumors. During the last several years, a number of radiolabeled RGD peptides have been evaluated as integrin ![]() -targeted radiotracers by single photon emission computed tomography (SPECT) or positron emission tomography (PET) in pre-clinical animal models and human clinical trials.
-targeted radiotracers by single photon emission computed tomography (SPECT) or positron emission tomography (PET) in pre-clinical animal models and human clinical trials.
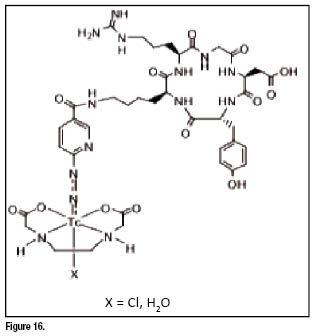
Cyclic RGD peptides, such as c(RGDfK) and ![]() , have been used to develop Tc-99m radiotracers for imaging integrin
, have been used to develop Tc-99m radiotracers for imaging integrin ![]() expression in tumors [66,67]. The results from biodistribution studies showed that
expression in tumors [66,67]. The results from biodistribution studies showed that ![]() (figure 16) has the best biodistribution characteristics in terms of tumor uptake, tumor/liver and tumor/lung ratios. RP593 showed much higher tumor uptake and longer retention than [
(figure 16) has the best biodistribution characteristics in terms of tumor uptake, tumor/liver and tumor/lung ratios. RP593 showed much higher tumor uptake and longer retention than [![]() (HYNIC-c(RGDfK))(tricine)(TPPTS)] (RP582). The tumor uptake of RP593 can be blocked by co-injection of excess c(RGDfV), suggesting that its tumor localization is indeed due to the integrin
(HYNIC-c(RGDfK))(tricine)(TPPTS)] (RP582). The tumor uptake of RP593 can be blocked by co-injection of excess c(RGDfV), suggesting that its tumor localization is indeed due to the integrin ![]() binding. RP593 and RP582 were also evaluated in the female Balb/c mice with subcutaneously growing OVCAR-3 ovarian carcinoma xenografts [68,69]. The tumor uptake of RP593 was significantly higher than that of RP582 at different time points. These results strongly suggest that cyclic RGD dimer
binding. RP593 and RP582 were also evaluated in the female Balb/c mice with subcutaneously growing OVCAR-3 ovarian carcinoma xenografts [68,69]. The tumor uptake of RP593 was significantly higher than that of RP582 at different time points. These results strongly suggest that cyclic RGD dimer ![]() [70] has a significant advantage over its monomer counterpart with respect to the tumor uptake and retention of their
[70] has a significant advantage over its monomer counterpart with respect to the tumor uptake and retention of their ![]() radiotracers.
radiotracers.
REFERENCIAS BIBLIOGRÁFICAS
1. BOSCHI A, DUATTI A, UCCELLI L. Development of technetium-99m and rhenium-188 radiopharmaceuticals containing a terminal metal-nitrido multiple bond for diagnosis and therapy. Topics Curr. Chem. 2005; 252: 85-115.
2. DUATTI A, UCCELLI L. Technetium complexes and radiopharmaceuticals containing the TcN multiple bond. Trends Inorg. Chem. 1996; 4: 27-41.
3. PASQUALINI R, et. al. A new efficient method for the preparation of 99mTc-radiopharmaceuticals containing the TcN multiple bond. Appl. Radiat. Isot. 1992; 43(11): 1329-1333.
4. BOLZATI C, et. al. Geometrically controlled selective formation of nitrido technetium(V) asymmetrical heterocomplexes with bidentate ligands. J. Am. Chem. Soc. 2000; 122(8): 4510-4511.
5. REFOSCO F, et. al. Mixed-Ligand Tc- and Re-nitrido complexes for radiolabeling bioactive molecules. Recent Res. Devel. Inorganic Chem. 2000; 2: 89-98.
6. BOLZATI C, et. al. Chemistry of the strong electrophilic metal fragment [99Tc(N)(PXP)]2+ (PXP = diphosphine ligand). A novel tool for the selective labeling of small molecules. J. Am. Chem. Soc. 2002; 124(38): 11468-11479.
7. BOCHER M, et. al. A fast cardiac gamma camera with dynamic SPECT capabilities: design, system validation and future potential. Eur. J. Nucl. Med. Mol. Imaging. 2010; 37: 1887–1902.
8. HUTTON BF. New SPECT technology: potential and challenges. Eur. J. Nucl. Med. Mol. Imaging. 2010; 37: 1883–1886.
9. ALBERTO R, et. al. Synthesis and Properties of Boranocarbonate: A Convenient in Situ CO Source for the Aqueous Preparation of [99mTc(OH2)3(CO)3]+. J. Am. Chem. Soc. 2001; 123: 3135.
10. ALBERTO R. Radiopharmaceuticals. In: Bioorganometallics. Weinheim: Wiley-VCH, 2006. p 97.
11. ALBERTO R, et. al. A Novel Organometallic Aqua Complex of Technetium for the Labeling of Biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]- in Aqueous Solution and Its Reaction with a Bifunctional Ligand. J. Am. Chem. Soc. 1998; 120(31): 7987-7988.
12. LIU S. The role of coordination chemistry in the development of target-specific radiopharmaceuticals. Chem. Soc. Rev. 2004; 33(7): 445-61.
13. THOMPSON KH, ORVIG C. Metal complexes in medicinal chemistry: new vistas and challenges in drug design. Dalton Trans. 2006; 14(6): 761-4.
14. LA BELLA R, et. al. In vitro and in vivo evaluation of a Tc-99m(I)-labeled bombesin analogue for imaging of gastrin releasing peptide receptor-positive tumors. Nucl. Med. Biol. 2002; 29(5): 553-560.
15. VEERENDRA B, et. al. Synthesis, radiolabeling and in vitro GRP receptor targeting studies of (99m) Tc-Triaza-X-BBN[7-14]NH2 (X = serylserylserine, glycylglycylglycine, glycylserylglycine, or beta alanine). Synth. React. Inorg. Me. 2006; 36: 481-491.
16. SMITH CJ, et. al. Radiochemical investigations of gastrin-releasing peptide receptor-specific [Tc-99m(X)(CO)(3)-Dpr-Ser-Ser-Ser-Gln-Trp-Ala-Val-Gly-His-Leu-Met-(NH2)] in PC-3, tumor-bearing, rodent models: Syntheses, radiolabeling, and in vitro/in vivo studies where Dpr=2,3-diaminopropionic acid and X = H2O or P(CH2OH)(3). Cancer Res. 2003; 63 (14): 4082-4088.
17. WAIBEL R, et. al. Stable one-step technetium-99m labeling of His-tagged recombinant proteins with a novel Tc(I)-carbonyl complex. Nat. Biotechnol. 1999; 17(9): 897-901.
18. FERREIRA CL, et. al. Glucosamine Conjugates of Tricarbonylcyclopentadienyl Rhenium(I) and Technetium(I) Cores. Inorg. Chem. 2006; 45(17): 6979-6987.
19. FERREIRA, et. al. Carbohydrate-appended 3-hydroxy-4-pyridinone Complexes of the [M(CO)(3)](+) core (M) Re, Tc-99m, Re-186). Bioconjugate Chem. 2006; 17(5): 1321-1329.
20. SCHIBLI R, et. al. Synthesis and in vitro characterization of organometallic rhenium and technetium glucose complexes against glut 1 and hexokinase. Bioconjugate Chem. 2005; 16(1): 105-112.
21. BERNARD J, et. al. Aqueous Synthesis of Derivatized Cyclopentadienyl Complexes of Technetium and Rhenium Directed Toward Radiopharmaceutical Application. Inorg. Chem. 2003; 42(4): 1014-1022.
22. TZANOPOULOU S, et. al. Synthesis, Characterization, and Biological Evaluation of M(I)(CO)(3)(NNO) Complexes (M = Re, Tc-99m) Conjugated to 2-(4-aminophenyl)benzothiazole as Potential Breast Cancer Radiopharmaceuticals. J. Med. Chem. 2006; 49(18): 5408-10.
23. LIU Y, et. al. Amino Acids Labeled with [99mTc(CO)3]+ and Recognized by the L-Type Amino Acid Transporter LAT1. J. Am. Chem. Soc. 2006; 128(50): 15996-97.
24. STEPHENSON KA, et. al. Bridging the gap between in vitro and in vivo imaging: Isostructural Re and Tc-99m complexes for correlating fluorescence and radioimaging studies. J. Am. Chem. Soc. 2004; 126(29): 8598-99.
25. ALBERTO R. [Tc(CO)3]+ Chemistry: a Promising New Concept for SPET?. Eur. J. Nucl. Med. Mol. I. 2003; 30(9): 1299-1302.
26. WELCH M. [Tc(CO)3]+ chemistry: a promising new concept for SPET?. Eur. J. Nucl. Med. Mol. Imaging. 2003; 30(9): 1302-1304.
27. GARCIA R, PAULO A, DOMINGOS A, et. al. Re and Tc complexes containing B-H...M agostic interactions as building blocks for the design of radiopharmaceuticals. J. Am. Chem. Soc. 2000; 122(45): 11240-11241.
28. WALD J, et. al. Aqueous One-Pot Synthesis of Derivatized Cyclopentadienyl-Tricarbonyl Complexes of 99mTc with an In Situ CO Source: Application to a Serotonergic Receptor Ligand. Angew. Chem. Int. Ed. 2001; 40(16): 3062-3066.
29. ALBERTO R, et. al. Mono-, Bi-, or Tridentate Ligands? The Labeling of Peptides with Tc-99m-Carbonyls. Biopolymers. 2004; 76(4): 324-333.
30. PASQUALINI R, et. al. Bis(dithiocarbamato) nitrido technetium-99m radiopharmaceuticals: a class of neutral myocardial imaging agents. J. Nucl. Med. 1994; 35(2): 334-341.
31. PASQUALINI R, DUATTI A. Synthesis and characterization of the new neutral myocardial imaging agent [99mTcN(noet)2] (noet = N-ethyl-N-ethoxydithiocarbamato). J. Chem. Soc. Chem. Commun. 1992; (18): 1354-1355.
32. JOHNSON G, et. al. Clearance of technetium-99m N-NOEt in normal, ischemic-reperfused, and membrane-disrupted myocardium. J. Nucl. Cardiol. 1996; 3(1): 42-54.
33. GIGANTI M, et. al. Advances in radiopharmaceuticals for cardiac imaging: 99mTcN-NOET. Q. J. Nucl. Med. 1997; 41(Suppl.): 147-151.
34. TAKEHANA K, et. al. Assessment of residual coronary stenoses using 99mTc-N-NOET vasodilator stress imaging to evaluate coronary flow reserve early after coronary reperfusion in a canine model of subendocardial infarction. J. Nucl. Med. 2001; 42(9): 1388-1394.
35. VANZETTO G, et. al. 99mTc-N-NOET myocardial uptake reflects myocardial blood flow and not viability in dogs with reperfused acute myocardial infarction. Circulation. 2000; 101(20): 2424-2430.
36. HOLLY TA, et. al. The effect of ischemic injury on the cardiac transport of Tc-99m-N-NOET in the isolated rabbit heart. J. Nucl. Cardiol. 1999; 6(6): 633-640.
37. VANZETTO G, et. al. Myocardial uptake and redistribution of 99mTc-N-NOET in dogs with either sustained coronary low flow or transient coronary occlusion: comparison with 201Tl and myocardial blood flow. Circulation. 1997; 96(7): 2325-2331.
38. JEETLEY P, et. al. Comparison between Tc-99m-N-NOET and Tl-201 in the assessment of patients with known or suspected coronary artery disease. J. Nucl. Cardiol. 2004; 11(6): 664-672.
39. PETRUZELLA FD, et. al. Optimal timing for initial and redistribution technetium-99m-N-NOET image acquisition. J. Nucl. Cardiol. 2000; 7(2): 123-131.
40. VANZETTO G, et. al. Biodistribution, dosimetry, and safety of myocardial perfusion imaging agent 99mTcN-NOET in healthy volunteers. J. Nucl. Med. 2000; 41(1): 141-148.
41. CALNON DA, et. al. Myocardial uptake of 99mTc-N-NOET and 201Tl during dobutamine infusion. Comparison with adenosine stress. Circulation. 1999; 100: 1653-1659.
42. JOHNSON G, et. al. Planar imaging of 99mTc-labeled (bis(N-ethoxy, N-ethyl dithiocarbamato) nitrido technetium(V) can detect resting ischemia. J. Nucl. Cardiol. 1997; 4(3): 217-225.
43. SINUSAS AJ. Technetium 99m-N-NOET: although not equivalent to thallium-201, it still offers new opportunities. J. Nucl. Cardiol. 2000; 7(2): 185-188.
44. JOHNSON G, et. al. Interaction of technetium-99m-N-NOET with blood elements: potential mechanism of myocardial redistribution. J. Nucl. Med. 1997; 38(1): 138-143.
45. JOHNSON G, et. al. Clearance of technetium 99mTcN-NOET in normal, ischemic-reperfused, and membrane-disrupted myocardium. J. Nucl. Cardiol. 1996; 3(1): 42-54.
46. UCCELLI L, et. al. Subcellular distribution of technetium-99mTcN-NOEt in rat myocardium. J.Nucl. Med. 1995; 36(11): 2075-2079.
47. BOSCHI A, et. al. Synthesis and biological evaluation of monocationic asymmetric 99mTc-nitride heterocomplexes showing high heart uptake and improved imaging properties. J. Nucl. Med. 2003; 44(5): 806-814.
48. OSCHI A, BOLZATI C, UCCELLI L, et. al. A class of asymmetrical nitrido 99mTc heterocomplexes as heart imaging agents with improved biological properties. Nucl. Med. Commun. 2002; 23(7): 689-693.
49. HATADA K, et. al. 99mTc-N-DBODC5, a new myocardial perfusion imaging agent with rapid liver clearance: comparison with 99mTc-sestamibi and 99mTc-tetrofosmin in rats. J. Nucl. Med. 2004; 45(12): 2095-2101.
50. BOLZATI C, et. al. Subcellular distribution and metabolism studies of the potential myocardial imaging agent [99mTc(N)(DBODC)(PNP5)]+. J. Nucl. Med. 2008; 49(8): 1336-1344.
51. ZHANG WC, et. al. Experimental study of [99mTc(PNP5) (DBODC)]+ as a new myocardial perfusion imaging agent. Cardiology. 2009; 112(2): 89-97.
52. HATADA K, et. al. Organ biodistribution and myocardial uptake, washout, and redistribution kinetics of Tc-99m N-DBODC5 when injected during vasodilator stress in canine models of coronary stenoses. J. Nucl. Cardiol. 2006; 13(6): 779-790.
53. CITTANTI C, et. al. Whole-body biodistribution and radiation dosimetry of the new cardiac tracer 99mTc-N-DBODC. J. Nucl. Med. 2008; 49(8): 1299-1304.
54. KIM YS, et. al. Tc-99m-N-MPO: novel cationic Tc-99m radiotracer for myocardial perfusion imaging. J. Nucl. Cardiol. 2008; 15(4): 535-546.
55. KIM YS, et. al. Mechanism for myocardial localization and rapid liver clearance of Tc-99m-N-MPO: a new perfusion radiotracer for heart imaging. J. Nucl. Cardiol. 2009; 16(4): 571-579.
56. MARIA L, et. al. Tris(pyrazolyl)methane 99mTc tricarbonyl complexes for myocardial imaging. Dalton Trans. 2009; 28(4): 603-606.
57. MARIA L, et. al. Rhenium and technetium tricarbonyl complexes anchored by pyrazole-based tripods: novel lead structures for the design of myocardial imaging agents. Dalton Trans. 2007; 28(28): 3010–3019.
58. BLUM JE, HANDMAKER H, RINNE N A. The utility of a somatostatin-type receptor binding peptide radiopharmaceutical (P829) in the evaluation of solitary pulmonary nodules. Chest 1999; 115(1): 224-232.
59. CYR JE, et. al. Somatostatin receptor-binding peptides suitable for tumor radiotherapy with Re-188 or Re-186. Chemistry and initial biological studies. J. Med. Chem. 2007; 50 (6): 1354-1364.
60. DECRISTOFORO C, et. al. 99mTc-HYNIC-[Tyr3]-octreotide for imaging somatostatin-receptor-positive tumors: preclinical evaluation and comparison with 111In-octreotide. J. Nucl. Med. 2000; 41(6): 1114-1119.
61. DECRISTOFORO C, et. al. 99mTc-EDDA/HYNIC-TOC- A new technetium-99m labelled radiopharmaceutical for imaging somatostatin receptor positive tumours: first clinical results and intra-patient comparison with 111In-labelled octreotide derivatives. Eur. J. Nucl. Med. 2000; 27(9): 1318-1325.
62. GABRIEL M, et. al. An intrapatient comparison of 99mTc-EDDA/HYNIC-TOC with 111In-DTPA-octreotide for diagnosis of somatostatin receptor-expressing tumors. J. Nucl. Med. 2003; 44(5): 708-716.
63. FOLKMAN J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002; 29(6 Suppl. 6): 15-18.
64. HWANG R, VARNER J. The role of integrins in tumor angiogenesis. Hematol. Oncol. Clin. North. Am. 2004; 18(5): 991-1006.
65. LIU S, ROBINSON SP, EDWARDS DS. Radiolabeled integrin avb3 antagonists as radiopharmaceuticals for tumor radiotherapy. Topics Curr. Chem. 2005; 252: 193-216.
66. LIU S. 99mTc-Labeling of a hydrazinonicotinamide-conjugated vitronectin receptor antagonist useful for imaging tumors. Bioconjug. Chem. 2001; 12(4): 624-629.
67. LIU S, et. al. Effect of coligands on biodistribution characteristics of ternary ligand 99mTc complexes of a HYNIC-conjugated cyclic RGDfK dimer. Bioconjug. Chem. 2005; 16(6): 1580-1588.
68. LIU S, et. al. Evaluation of a 99mTc-labeled cyclic RGD tetramer for non-invasive imaging integrin avb3-positive breast cancer. Bioconjug. Chem. 2007; 18(2): 438-446.
69. LIU S, et. al. Impact of PKM linkers on biodistribution characteristics of the 99mTc-labeled cyclic RGDfK dimer. Bioconjug. Chem. 2006; 17(6): 1499-1507.
70. DECRISTOFORO C, et. al. [99mTc]HYNIC-RGD for imaging integrin avb3 expression, Nucl. Med. Biol. 2006; 33: 945-952.
Recibido: 30 de agosto de 2012
Aceptado: 4 de octubre de 2012