Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent form of liver disease in the USA, affecting an estimated 30% of the population and associated with metabolic syndrome.1,2 The severe form of NAFLD is non-alcoholic steatohepatitis (NASH), which has a profound relationship with overweight, obesity, and metabolic syndrome. An unhealthy lifestyle and diet, and decreased physical activity play an important role in the development and progression of NAFLD.3,4 Also, the role of intestinal microflora in the development of metabolic disorders and metabolic-associated liver damage, which is NASH, has been proven.5,6,7,8
Key development mechanisms of NASH are the accumulation of free fatty acids, triglycerides, and activation of lipid peroxidation in the liver, which leads to the toxic intermediate products build-up that stimulate the development of inflammation and fibrotic lesion.3,5,9
A basic condition for the treatment and prevention of NASH is a low-calorie diet, restricted in saturated fatty acids (SFA), cholesterol, fructose, and simple carbohydrates, but high in polyunsaturated fatty acids (PUSFA), pre- and probiotics, and natural antioxidants.3,5,6,7,10 In this regard, there is an opinion, that mare's milk (Saumal) is a perfect finding in NASH diet therapy. Saumal is not only an optimal dietary supplement but also has a high therapeutic potential generally in NAFLD.
The healing qualities of mare's milk have been known for a long time.11,12,13,14,15,16,17,18,19 In various countries of the world, mare's milk is positioned as an effective remedy for problems with digestion and liver.11,12,13 Mare's milk possesses not only nutritional but also medicinal properties. Its regular use relieves or eliminates the symptoms of diseases of the digestive system, it is effective in chronic liver diseases, including hepatitis.11,12,13
The study was carried out with the use of Saumal freezedried mare's milk (SMM) produced by “Eurasia Invest Ltdˮ LLP (Karaganda region, Kazakhstan), where large-scale production of SMM was established with the participation of the German horse breeding farm Hans Zollmann using an innovative freeze drying technology in strict compliance with the European quality standard.11,12,20
The study had the aim of assessing the effectiveness of the use of mare's milk in NASH based on clinical, biochemical, and fibroelastometric studies.
Methods
The work was carried out in 3 clinical centers: the Corporate Foundation “Nazarbayev University Medical Centerˮ (Nur-Sultan, Kazakhstan), the Hospital of the Medical Center of the Department of Presidential Affairs of the Republic of Kazakhstan (Nur-Sultan, Kazakhstan), and at the Department of Internal Medicine of the Kazakh-Russian Medical University (Almaty, Kazakhstan), possessing all the necessary conditions for conducting this scientific research.
Within the framework of this study, the ethical principles of conducting biomedical research of the Republic of Kazakhstan, national and international guidelines on the ethics of research with human participants as a trial subject are followed. The ethical principles of the Declaration of Helsinki for the conduct of medical research with the participation of a human as a research subject are observed. Preliminary approval of the local ethical committee of the Corporate Foundation “University Medical Centerˮ LLC “Nazarbayev Universityˮ dated September 25, 2017, was received.
The study was carried out on 74 NASH patients aged 36 to 60 years after the prior informed consent of all patients. The study involved 35 men and 39 women. The study is multicenter, open-label, comparative, controlled, and randomized. As a result of randomization, the patients were divided into three groups:
main group 1 (n = 28) - NASH patients who took only Saumal freeze-dried mare's milk;
main group 2 (n = 27) - NASH patients who took SMM "Saumal" in combination with ursodeoxycholic acid (UDCA "Ursosan"),
control group (n = 19) - NASH patients who took ursosan as monotherapy as hepatoprotection.
To study clinical symptomatology, the main NASH syndromes were selected, namely: asthenic, pain, and dyspeptic, and for the study of laboratory parameters-transaminases (ALT, AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), bilirubin, cholesterol, low density lipoproteins (LDL), high density lipoproteins (HDL), triglycerides (TG) and glucose. Biochemical blood tests were performed on an “Architectc 8000ˮ biochemical analyzer (Abbott, USA). For greater clarity, biochemical studies of NASH patients were carried out after 2 weeks, and after a month from the start of treatment, and at the end of treatment.
Among other things, investigated were the standard ultrasound parameters of the hepatobiliary system before and after the end of the reception of SMM “Saumalˮ. Ultrasound examinations of the liver, biliary вгсеы, pancreas, and spleen were carried out using ultrasound scanners with an abdominal sensor from well-known companies Philips (Netherlands), Sony (Japan), Siemens (Germany). The study was carried out before the start of treatment and at the end of treatment.
Fibroelastometry of liver tissue to determine the stage of fibrosis and the degree of steatosis was carried out on a “Fibroscan 502 Touch CAPˮ unit (France) fitted with an ultrasound transducer for non-invasive determination of the stage of fibrosis by the method of transient elastometry and by measuring the attenuation of ultrasound: Controlled Attenuation Parameter (CAPTM) - to determine the degree of steatosis. Fibroelastometry was also performed before and 2 months after the commencement of treatment.
Statistical analysis was performed using SPSS for Windows version 23.0 (SPSS: IBM, Armunk, NY) and Microsoft Excel-2013. A two-way t-test and odds ratio (OR) with 95% confidence intervals were used. The value p < 0.05 was found to be significant.
The work was carried out within the scope of a scientific grant (URN AR051355855) of the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan on the priority "Science of Life" after obtaining the preliminary consent of patients for the study and the conclusions of the local ethical committee. The scientific project is registered in the ClinicalTrials.gov database.
Results and Discussion
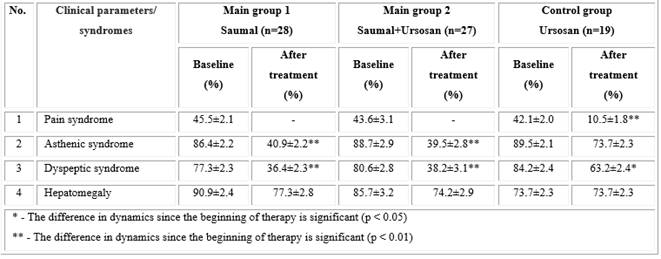
When conducting clinical, laboratory, and instrumental studies in NASH patients, an improvement in the clinical symptomatology of the disease was noted in all treated patients, but more significant improvements were found in the main groups 1 and 2 (Table 1). Thus, pain syndrome in the form of a feeling of heaviness and discomfort in the right hypochondrium, which is present in 45.5 ± 2.1% and 43.6 ± 3.1% of patients in main groups 1 and 2, respectively, after the end of 2 months of taking "Saumal" (main group 1) and "Saumal + Ursosan" (main group 2) did not disturb the patients. In the control group, this syndrome persisted after treatment in 10.5 ± 1.8% of patients (p < 0.01).
After the course of treatment in the main groups 1 and 2, asthenic and dyspeptic syndromes significantly decreased (p < 0.01), and in the control group, the decrease in these parameters was insignificant compared to the main group: asthenic syndrome decreased insignificantly (p > 0.05), whereas decline of the dyspeptic syndrome was statistically significant, but less profound than in the main groups (p < 0.05).
Hepatomegaly detected based on ultrasound examination (US) before treatment with "Saumal" and "Saumal + Ursosan" was observed in 90.9 ± 2.4% and 77.3 ± 2.8% of patients, respectively, and after the therapy its frequency decreased by 13.6% and 11.5%, amounting to 77.3 ± 2.8% and 77.3 ± 2.8%, respectively (p > 0.05). A study in the control group demonstrated that "Ursosan" alone had little or no effect on hepatomegaly.
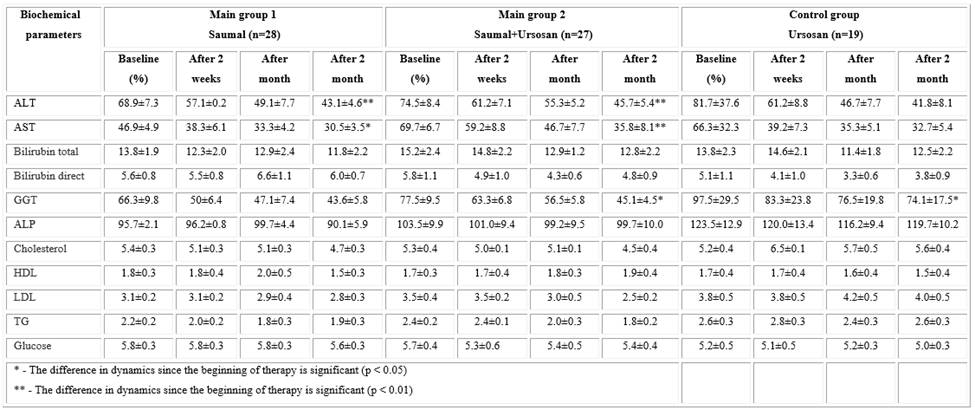
In laboratory research, an improvement in biochemical parameters in the studied groups was also revealed, but more pronounced changes were observed in experimental groups 1 and 2 (Table 2). Thus, in these groups, all biochemical parameters gradually decreased or normalized over the course of 2 months while taking Saumal in combination with UDCA. The ALT and AST cytolysis indices significantly decreased relative to the beginning of treatment both in the main group 1 (p < 0.01 and
p < 0.05, respectively) and in the "Saumal + Ursosan" group (p < 0.01 in both groups). Cytolysis indices in the Ursosan control group also decreased compared to the beginning of treatment but were statistically insignificant (p > 0.05).
Moreover, in all 3 investigation groups, there was a decrease in such indicators of cholestasis as GGT and ALP. Moreover, in the groups "Saumal + Ursosan" and "Ursosan", the decrease in GGT was statistically significant (p < 0.05), which confirms the anticholestatic effect of UDCA. The decrease in ALP in all groups at the end of treatment was not statistically confirmed (p > 0.05).
A very important and challenging property of mare's milk is its positive effect on cholesterol exchange. In this study, a decrease in cholesterol, LDL, and TG in the Saumal and Saumal + Ursosan groups was observed, probably due to the cholesterol-lowering and bifidogenic properties of Saumal (Table 2). In the control group of patients, no change in these parameters was observed, which is typical for UDCA.
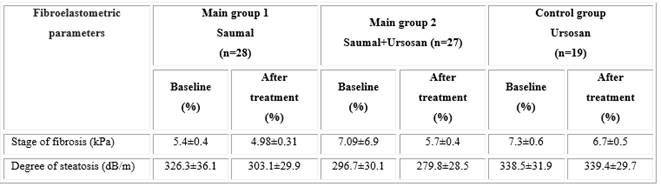
Fibroelastometric examination of liver tissue using the "Fibroscan 502 TouchCAP" unit revealed different dynamics of fibrosis and steatosis indicators during therapy (Table 3). In the main groups of studies 1 and 2, a decrease in the degree of liver steatosis by 23.2 and 16.9 dB/m, respectively, was revealed. These changes were statistically insignificant, but there is an opinion, they were quite considerable when taking mare's milk for only 2 months. For comparison, in the control group “Ursosanˮ there was no decrease in steatosis, but on the contrary, there was a certain tendency to its increasing. Also, in all studied groups, there was a tendency towards a decrease in liver fibrosis.
Thus, the conducted clinical study showed a significant improvement in the clinical-biochemical, ultrasound, and fiboelastometric parameters of NASH against the background of taking SMM "Saumal". At the same time, a comparative study showed that the effectiveness of mare's milk was higher than the effectiveness of conventional ursodeoxycholic acid (Ursosan), which is included in the clinical guidelines and protocols for the management of NAFLD as an effective hepatoprotective agent.4,21,22 Besides, some randomized clinical trials have shown a positive result from the use of UDCA in NASH,9,23,24,25,26 in which the severity of inflammation syndrome and hepatic steatosis decreased.
Taking mare's milk not only normalized liver biochemical parameters but also decreased cholesterol metabolism (total cholesterol, LDL, TG), the degree of liver steatosis, and existing hepatomegaly declined too. This pleiotropic effect of mare's milk points to the pathogenetic feasibility for the use of Saumal in NAFLD, including NASH.
Apart from the aforesaid, there are conflicting opinions in the literature regarding the effectiveness of UDCA in NAFLD.27,28 According to these researchers, the use of UDCA did not have a significant effect on inflammation, steatosis, and liver fibrosis in NASH. The ineffectiveness of ursodeoxycholic acid in NASH has also been mentioned in European and American clinical guidelines.29,30 It follows from these protocols that the use of UDCA in NASH for 2 years did not lead to a decrease in inflammation and an improvement in the histological picture of the liver compared to control. These facts once again confirm the effectiveness of the use of mare's milk in this pathology in comparison with UDCA.
Conclusions
Mare's milk is a more effective therapeutic and prophylactic agent for NASH in comparison with ursodeoxycholic acid, which is included in Kazakhstan and Russian clinical treatment protocols. At the same time, Saumal not only developed hepatoprotective properties but also revealed its hypocholesterolemic and lipotropic effects. Considering the existing pleiotropic effect of mare's milk, we believe that its use in NASH is pathogenetically justified, and Saumal can take its rightful place in clinical guidelines and protocols for the treatment of NAFLD as a therapeutic and prophylactic agent.