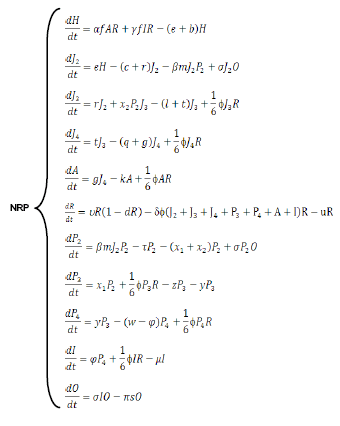Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Protección Vegetal
versión impresa ISSN 1010-2752
Rev. Protección Veg. vol.29 no.2 La Habana Mayo.-ago. 2014
TECHNICAL NOTE
A mathematic model for the interaction between Meloidogyne spp. and Pasteuria penetrans
Modelo matemático de la interacción entre Meloidogyne spp. y Pasteuria penetrans
Ileana Miranda, Lucila Gómez, Hugo L. Benítez, Yoannia Castillo, Dainé Hernández-Ochandía, Mayra G. Rodríguez
Centro Nacional de Sanidad Agropecuaria (CENSA), Apartado 10, San José de las Lajas, Mayabeque, Cuba. Email: ileanam@censa.edu.cu.
ABSTRACT
Some aspects of Meloidogyne spp. (root-knot nematode) growth regulation by the gram-negative bacterium Pasteuria penetrans were reviewed. The study allowed the construction of a mathematical model to predict the dynamics of both organisms. The model includes 11 differential equations and 31biological constants describing the life-cycle of the nematode and its relationship with the bacterium. To simulate the real behavior of the populations, the constants, which represent biological parameters, have to be evaluated under controlled conditions similar to those of soil microcosm.
Key words: bio-mathematics, Meloidogyne, Pasteuria penetrans, biological control.
RESUMEN
El estudio de algunos aspectos de la regulación del crecimiento de los nematodos agalleros del género Meloidogyne Goeldi por la acción de la bacteria Pasteuria penetrans, permitió proponer un modelo matemático para predecir la dinámica de ambos organismos. El modelo incluye 11 ecuaciones diferenciales y 31 constantes biológicas que describen las fases de desarrollo del nematodo y su relación con la bacteria. Para simular el comportamiento real de las poblaciones, las constantes, que representan parámetros biológicos, deberán ser evaluadas en condiciones controladas similares a la del microcosmo del suelo.
Palabras clave: biomatemática, Meloidogyne, Pasteuria penetrans, control biológico.
Root-knot nematodes (Meloidogyne spp.) are severe pests of food crops in many agricultural systems, worldwide. They produce several economic losses when feeding on plants roots (1). To counteract their effects, different control practices (chemical, cultural, including resistance, physical barriers, and others) have been used; however, the practical use of biological control agents has been recently introduced (2).
The bacteria Pasteuria penetrans is an endospore-forming parasite of nematodes, and has shown a great potential as a biological control agent of root-knot species. This bacterium has been reported to provide an effective control when used alone or combined with other tactics, both in the open field (3) and in greenhouses or micro-plots (4, 5).
In the last years, considerable progress have been achieved in the studies on P. penetrans contributing to understand its biology and importance as a biocontrol agent, and the knowledge regarding its ecology, life-cycle and host adhesion mechanisms has increased (6).
P. penetrans parasitism begins with the adhesion of one to hundreds endospores to the infective stage of the nematode (second stage juveniles) (J2), where they germinate through the nematode cuticle (7). Penetration of J2s into the root decreases according to the number of spore adhered (8). After parasitized, the nematode continues with the endospores adhered until becomes adult female, when the endospores are released to the soil.
Nevertheless, there are some questions concerning the proportion in which the development rate of the nematode population may be affected once the endospores have adhered to the J2s, and understanding the factors which influence the nematode population reduction is important to increase the efficacy of the bacterium as a biocontrol agent. A mathematical model describing a density-dependent relationship with the nematode as a host for the bacteria can help to solve this query (9), keeping in mind that the parameters involved will need to be experimentally determined for each soil microcosm, also considering the density-dependence factors not been included in the implementation of the model.
The model was constructed from the basic nematode life-cycle (10), incorporating what was known about its interactions with P. penetrans (11). The eggs (H) hatch (at rate e) yielding immature stages (infestive juveniles, J2), which after going through stages J3 and J4, eventually become females without bacterial infection, which produce eggs at rate a at a fertility level. The nematodes without endospore encumbrance can die because of the abrupt changes in temperatures or by an interruption in the complete cycle of the crop. From the holistic point of view the population level of any Meloidogyne sp. is the product of the food supply, adaptation to the physical and biological environment and the compatibility of the plant and nematode (12). For that, the model includes different constants indicating a natural mortality (r, l, q, and k). Due to the matching (at transmission rate b) of the J2 with the P. penetrans endospores released in soil (at rates) by nematode infected females, some J2 nematodes are unable to enter the root system due to endospore encumbrance, whereas others can complete the cycle (P2, P3, P4) until becoming infested females (I), which can still produce a minor number of eggs (at rate ) and then release news endospores. Some of these endospores can be eliminated by the effect of some ecosystem factors(s subtraction factor), for example the runoff water, or can adhere to the J2s, bearing or not endospores. A small quantity of J2s (x2) withless than three spores adhered is able to pass to the third larval stage without infestation (J3), because the spores do not germinate. Although the endospores can persist for many years in soil, they have a certain mortality rate (p).The J2s that are able to penetrate the root produce a certain degree of damage in the plant root, and other nematode stages (J3, J4, A) produce a negative effect on the plant too (Figure).
The biological parameters were considered as constants because the external factors were not included (Table). These constants are specific to any soil microcosm and have to be calculated under experimental controlled conditions considering the optimum temperature for growth and reproduction of Meloidogyne spp. is 25-30°C (12).
Soil texture and structure, as well as the soil matrix, are important factors that act upon the flow and adsorption of the spores (13). Soil with 10-30% of clay is the ideal soil for the best balance of adsorption and retention of the P. penetrans endospores. The interactions occurring in this soil may exert direct control on the dispersion and adhesion capacity of the endospores to the nematode cuticle. The irrigation level of the soil must also be known, since with an appropriate irrigation, the nematode populations increase and an increasing number of endospores adhere to J2 cuticles. However, an intense irrigation can cause the endospores to be lost due to dilution and percolation to deeper soil layers (14).
Another important factor is soil pH, whose effects on the nematode and bacteria are diverse and of difficult interpretation. Meloidogyne spp. survives, hatch and reproduce over a wide pH range, 4-8. As with other soil solution factors, pH fluctuates with changes in soil moisture and soil salinity. As with soil texture, increased crop damage from Meloidogyne spp. is often associated with alkaline soils (12). Apparently favorable pH for endospore adhesion to the host nematodes oscillates from neuter to alkaline too (15).
To estimate the parameters that allow simulating the dynamics under optimal conditions, it is necessary to know not only the favorable conditions to P. penetrans, but also those favorable conditions for the nematode growth and development of the host crop.
The biological model can be simulated using a system of differential equations:
In a further study, the parameters of the model will be estimated and the system stability analyzed using the Lapuniov´s theorem (16). Simulations, even when the evolution in time of a natural system cannot be «forecasted» due to its external perturbations and the presence of its own chaotic components, may allow understanding some details of the mechanisms of nematode natural regulation or suppression. The simulations of the NRP model will show that the behavior and dynamics of a simple system including a host and a bacterial parasite population are not only affected by the biological constants characterizing the two organisms, but also by the densities at which they occur. In some cases, changes in one or more constant/components of a model during a simulation (including the initial points used to start the model) may yield a cycle path leading to the extinction of one or both components, i.e. a local extinction may be considered when the cycle orbit becomes wide enough to reach one of the axes. Furthermore, by this way it is possible to estimate the doses and the time required to reach equilibrium between host and parasite, or to induce a local extinction, when routine treatments with biocontrol agents are possible (11).
ACKNOWLEDGEMENTS
First author thanks CNR (Italy) for a Short-Term Mobility Programme grant. Research partially funded by EU Project ICA4-CT-2002-10044, MicoSpa: Microbial pest control for sustainable peri-urban/urban agriculture in Latin America (Mexico and Cuba). We especially thank Aurelio Ciancio (IPP, CNR) for editing and suggestions and Dr. Eduardo Sistachs for his English revision.
REFERENCES
1. Moens M, Perry RN, Starr JL. Meloidogyne Species - a diverse group of novel and important Plant Parasites. En Moens M, RN Perry, JL Starr (Eds). CAB International. Root-knot Nematodes. 2009. Pp. 1-17.
2. Matielle T, Koumborb RD, Sabine F, Mamadou T. Host- parasite soil communities and environmental constraints: Modelling of soil functions involved in interactions between plant-parasitic nematodes and Pasteuria penetrans. Soil Biology & Biochemistry. 2010;42:1193-1199.
3. Tian B, Yang J, Zhang KQ. Transfer and Development of Pasteuria penetrans. J Nematol. 2007;39(1):55-61.
4. Darban DA, Pembroke Barbara, Gowen SR. The relationships of time and temperature to body weight and numbers of endospores in Pasteuria penetrans-infected Meloidogyne javanica females Nematology. 2004;6(1):33-36.
5. Javed N, El-Hassan S, Gowen S, Pemproke Barbara, Inam-ul -Haq M. The potential of combining Pasteuria penetrans and neem (Azadirachta indica) formulations as a management system for root-knot nematodes on tomato. Eur J Plant Pathol. 2008;120:53-60.
6. Gómez L, Gandarilla H, Rodríguez M. Pasteuria penetrans como agente de control biológico de Meloidogyne spp. Rev Protección Veg. 2010;25(3):137-149.
7. Bird DM, Opperman CH, Davies KG. Interactions between bacteria and plant-parasitic nematodes: now and then. Int J Parasitol. 2003;33:1269-1276.
8.Davies KG, Fargette M, Balla G. Cuticle heterogeneity as exhibited by Pasteuria spore attachment is not linked to the phylogeny of parthenogenetic root-knot nematode (Meloidogyne spp.). Parasitology. 2000;122:111-120.
9. Ciancio A, Bourijate M. Relationship between Pasteuriapenetrans infection levels and density of Meloidogyne javanica. Nematol Medit. 1995;23:43-49.
10.Hernández-Ochandía D, Arias Y, Gómez L, Peteira B, Miranda I, Rodríguez M. Elementos del ciclo de vida de población cubana de Meloidogyne incognita (Kofoid y White) Chitwood en Solanum lycopersicum L. Rev Protección Veg. 2012;27(3):188-193.
11.Ciancio A. Modeling nematodes regulation by bacterial endoparasites. En Mukerji K.G. (eds.). Springer. Integrated Management and Biocontrol of Vegetable and Grain Crops Nematodes. 2008. Pp 321-337.
12.Van Gundy SD. Ecology of Meloidogyne spp. Emphasis on environmental factors affecting survival and pathogenicity. En Sasser and Carter (eds). North Carolina Stage University Graphics. An Advanced Treatise on Meloidogyne Volume I: Biology and Control. 1985. Pp 177-182.
13.Dabiré KR, Ndiaye S, Mounport D, Mateille T. Relationships between abiotic soil factors and epidemiology of the biocontrol bacterium Pasteuriapenetrans in a root-knot nematode Meloidogynejavanica -infested Weld. Biological Control. 2007;40:22-29.
14.Dabiré KR, Mateille T. Soil texture and irrigation influence the transport and the development of Pasteuria penetrans, a bacterial parasite of root-knot nematodes. Soil Biol Biochem. 2004;36:539-543.
15.Davies KG. Interactions between nematodes and microorganisms: bridging ecological and molecular approaches. Adv Appl Microbiol. 2005;57:53-78.
16.Sharov AA. Course Quantitative Population Ecology. Department of Entomology Virginiatech, Blacksbug. 1999: 240p. Disponible en: http://www.ento.vt.edu/sharov/pop ecol/ (Consulta: 8-4-2013).
Recibido: 25-10-2013.
Aceptado: 28-3-2014.