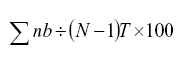Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Protección Vegetal
versión On-line ISSN 2224-4697
Rev. Protección Veg. vol.30 no.1 La Habana ene.-abr. 2015
ORIGINAL ARTICLE
Components of resistance to assess Black Sigatoka response in artificially inoculated Musa genotypes
Componentes de la resistencia para cuantificar la respuesta a la Sigatoka Negra en genotipos de Musa inoculados artificialmente
Michel Leiva-Mora*, Yelenys Alvarado-Capó, Mayra Acosta-Suárez, Mileidy Cruz-Martín, Berkis Roque, Eilyn Mena Méndez
Instituto de Biotecnología de las Plantas. Universidad Central «Marta Abreu» de Las Villas. Carretera a Camajuaní km 5.5. Santa Clara. Villa Clara. Cuba. CP 54 830. Fax: 53 (42) 281329; Tel: 53(42) 281257.
ABSTRACT
Components of resistance were evaluated in seven Musa genotypes artificially inoculated with mycelia fragment suspensions of Mycosphaerella fijiensis Morelet (strain CCIBP-Pf80). Incubation period, number and area of necrotic lesions, infection index, area under the disease progress curve, asexual latent period, and number of spermogonia were evaluated in the genotype leaves to dissect the infective cycle of the fungus under greenhouse conditions. Incubation period in the inoculated leaves began at 14-21 days post infection, and significant differences were detected in the response of the different genotypes. Necrotic lesions were only observed in Grande naine, Pisang Awak, and significantly less in Pisang lilin. Grande naine reached the highest percentage of leaflet area with necrotic tissue, followed by Pisang Awak and Pisang lilin. Grande naine and Pisang Awak reached the greatest areas under the disease progress curves, while the lowest values were calculated in FHIA-18 and FHIA-25. The asexual latent period in Grande naine and Pisang Awak was significantly shorter (approximately 14 days) than in Pisang lilin. Greater numbers of spermogonia were observed in Grande naine and Pisang Awak, followed by Pisang lilin. Conidia were only detected in Grande naine, Pisang Awak and, in a significantly less number, in Pisang lilin. The different response observed of Musa spp. genotypes to the causal agent of Black Sigatoka indicated that the components of resistance used allowed the quantitative assessment of their reaction to this fungus. These results could improve or facilitate the efficiency and precision of the early evaluation process in banana and plantain breeding programs.
Key words: early screening, inoculation, Mycosphaerella fijiensis, phytopathology, quantitative resistance.
RESUMEN
Se evaluaron los componentes de la resistencia en genotipos de Musa inoculados artificialmente utilizando suspensiones miceliales de Mycosphaerella fijiensis Morelet (cepa CCIBP-Pf80). El periodo de incubación, número de lesiones necróticas, área de lesiones necróticas, índice de infección, área bajo la curva del progreso de la enfermedad, periodo de latencia asexual y número de Espermogonios se evaluaron en siete genotipos para analizar el ciclo infectivo de M. fijiensis en condiciones de invernadero. El período de incubación en todos los genotipos se enmarcó entre 14-21 días posteriores a la inoculación y se observaron diferencias significativas en la respuesta de los diferentes genotipos de Musa. El mayor número de lesiones necróticas se observó en el genotipo Grande naine en comparación con el Pisang Awak, y este a su vez, respecto al Pisang lilin. Los mayores valores del área bajo la curva del progreso de la enfermedad se observaron en los genotipos Grande naine y Pisang Awak, mientras los menores se calcularon en FHIA-18 y FHIA-25. Grande naine y Pisang Awak tuvieron una reducción significativa del periodo de latencia asexual (aproximadamente 14 días) respecto al Pisang lilin. El mayor número de espermogonio se observó en Grande naine y Pisang Awak, seguido del Pisang lilin. Los conidios solo se detectaron en hojas necrosadas de Grande naine y Pisang Awak, las cuales fueron significativamente superiores al Pisang lilin. Las diferencias observadas en la respuesta de los genotipos de Musa spp. frente al agente causal de la Sigatoka Negra, indicaron que los componentes de la resistencia utilizados permitieron evaluar cuantitativamente la reacción de Musa spp. frente al agente causal de BLSD. Estos resultados podrían facilitar una mayor eficiencia y precisión de la evaluación temprana de genotipos de bananos y plátanos en programas de mejoramiento genético.
Palabras clave: fitopatología, inoculación, Mycosphaerella fijiensis, resistencia cuantitativa, selección temprana.
INTRODUCTION
Black Leaf Streak Disease (BLSD), caused by the ascomycete fungus Mycosphaerella fijiensis Morelet, is the causal agent of the disease also known as Black Sigatoka (1). This fungal disease is the most damaging and economically important disease of banana and plantain (Musa spp.) worldwide (1). This leaf pathogen is an increasing threat in all areas where Musa spp. are grown. Several toxins are produced by M. fijiensis (2) that may destroy the photosynthetic capacity of banana leaves causing reduced yield and premature ripening of the fruit (3). Fungicides are used to control BLSD, but they are expensive and not fully effective (1). Therefore, resistant genotypes are valuable to breeders and farmers and precise evaluation procedures are required (4, 5).
Measurements of Musa spp. germplasm resistance to M. fijiensis are often carried out in field trials under conditions of natural infestation, where ascospores and conidia are the main infective structures (6). Field evaluation are particularly time-consuming and costly because the plants have to be evaluated for several vegetative cycles and in different phenological stages, commonly affected by environment fluctuations (7).
Furthermore, tissue-culture-derived banana plants have been artificially inoculated with M. fijiensis and the disease development symptoms have been characterized in greenhouse and environmental growth chamber assays with stringent lighting and humidity controls (8), but without quantification of Musa response. However, the evaluation of the efficiency and durability of partial resistance in several genotypes of Musa have been performed by measuring some variables (size of lesion, number of perithecia and disease severity) in the life cycle of M. fijiensis at field and in detached leaf assay, but no greenhouse determination was done (9).
Nevertheless, the fungus cannot complete the entire infection cycle in vitro plantlets and detached leaf assay, due to the slow symptom development of M. fijiensis during the infection process. M. fijiensis symptoms can take up to two months or longer under optimal growth conditions, and normally senescent and not specific symptoms may be observed in that period. Although the response to BLSD has been evaluated by different qualitative ways, components of resistance in greenhouse conditions, with the mycelia fragment inoculation procedure, have never been used for this purpose.
As with other pathogen causing diseases, knowledge of the infective cycle under controlled condition is desirable to propose new quantitative variables and improve the effectiveness in evaluation of Musa breeding programs. The aim of the present study was to evaluate components of resistance to assess Black Sigatoka response in artificially inoculated Musa genotypes with mycelia fragment suspensions of M. fijiensis.
MATERIAL AND METHODS
Plant material and inoculation
Plants of seven Musa genotypes with different levels of resistance to BLSD, were obtained by tissue culture via organogenesis and acclimatized during 12 weeks. Calcutta 4 (AA) and Yangambi km 5 (AAA) were selected as resistant, Pisang lilin (AA), FHIA-18 (AAAB) and FHIA-25 (AAA) as partial resistant, while Grande naine (AAA) and Pisang Awak (ABB) as susceptible. Plants were planted in 0.5 L-plastic pots filled with humus, compost and zeolite mixture in a 5:3:2 v/v ratio.
All inoculation experiments were performed with a single-ascospore M. fijiensis isolate (strain CCIBP-Pf80) obtained from naturally infected banana leaves at stage 6 (10) from the susceptible cultivar Grande naine (AAA) showing typical symptoms of BLSD. This strain is preserved in the culture collection of M. fijiensis at the Applied Microbiology Laboratory of the Instituto de Biotecnología de las Plantas, Universidad Central «Marta Abreu» de Las Villas Carretera a Camajuaní km 5.5. Santa Clara, Villa Clara, Cuba. Ten plants per genotype were used for the artificial inoculation assay. Other five plants were not inoculated and left as control. Mycelia suspension was prepared following the protocol described by Leiva-Mora (11). Mycelial suspensions were filtered through sieves with a mesh of 40 µm and adjusted to 105 mycelia fragments.ml-1. Finally, gelatin at 1% (w/v) was added to improve adhesion of infective structures to the abaxial leaf surface.
The first three open leaves of each plant were inoculated on the abaxial leaf surface using a camel brush. Inoculated leaves were marked on the adaxial side with a black permanent marker. The plants were allowed to dry for two hours and humidity was maintained over 90% during the first three days by spraying water continuously. Afterwards, the humidity was saturated only during the night.
Components of resistance
The following components of resistance were evaluated separately in the third, second and first open leaves of the inoculated plants:
Incubation period (IP): Defined as the time elapsed between inoculation and symptom appearance in the inoculated plants of each genotype. IP was assessed daily by visual inspection of symptom development starting at 7 days post-inoculation (DPI).
Number of necrotic lesions and area of necrotic lesions (NNL and ANL)
NNL was counted in each inoculated leaves at 63 DPI. ANL was estimated in each inoculated leaves at 63 DPI by using the ellipse area formula (A = a/2. b/2 . p), where a was the length and b the width of the necrotic spot, and p=3.14.
Infection index (II): The percentage of leaflet area with necrotic tissue was estimated using a seven degree diagrammatic scale (12) modified (13) for BLSD resistance in Musa spp. The scale degrees were 0= no symptoms; 1= presence of stages 1, 2, or 3, but not more than 10 stage 4 symptoms; 2= more than 10 stage 4 but less than 5% of the leaf affected; 3= from 6 to 15% of the leaf affected; 4= from 16 to 33% of the leaf affected; 5= from 34 to 50% of the leaf affected; and 6 = more than 50% of the leaf affected).
Disease severity assessments were calculated at 63 DPI by the expression:
where n= number of leaves at each level; b= value of severity according to the diagrammatic scale, N= 7, corresponding with the number of stages in the scale and T= total number of leaves evaluated per plant.
Three inoculated leaves on each plant were evaluated.
Area under the disease progress curve (AUDPC): AUDPC was calculated according to Shaner and Finney`s (14) formula:
where Yi =BLSD severity (per unit), Xi =time (days) at the ith observation and n=total number of observations, previously infection index was determined by the evaluating scale (15) (Table 1).
Asexual latent period (ALP): ALP referred to the days elapsed from inoculation to the occurrence of conidia sporulation. Detection of conidia was performed according to Aguirre (16), and the evaluations were done weekly beginning at 35 DPI until 63 DPI. ALP was considered completed when sporulation was observed on at least three lesions on each inoculated leaf.
Number of spermogonia
Discs (1 cm of diameter) from leaves with necrotic lesions (stage 4 and 5 in the evaluation scale (15) were decolorized in 10% (w/v) KOH for 24h. They were washed in deionized steril water three times. The discs were placed on slides with lactophenol (phenol 20 g; lactic acid 20 g; glycerol 40 g; water 20 mL) and mounted for their further observation under the microscope (Olympus) with 200x magnification. One hundred observations of each Musa genotype were done under the microscope with 200x magnification and the total number of spermogonia recorded.
Statistical analysis
All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences Version 15.0) software (SPSS Inc., Chicago, IL, USA). The number of necrotic lesions, area of necrotic lesions, area under disease progress curve (AUDPC), number of spermogonia and conidia were compared according to Kruskall-Wallis non-parametric test. Infection index and asexual latent period were processed by One-way ANOVA and the means compared by Duncan`s test.
RESULTS AND DISCUSSION
Incubation period (IP)
The incubation period in all the inoculated leaves of the same genotypes began at 14-21 DPI, and significant differences were observed among the Musa genotype response (Table 2; Fig 1). FHIA-18 and FHIA-25 had the longest incubation period, followed by Pisang lilin. Calcutta 4, Grande naine, Pisang Awak and Yangambi were the genotypes with the shortest incubation period.
IP was found to be important because it showed significant differences among Musa artificially inoculated genotypes with mycelial suspension in greenhouse assays. It was the first time that incubation period has differentiated genotypes with differential response to Black Sigatoka in greenhouse using mycelial fragments. Similar results were obtained by Molina-Tirado and Castaño-Zapata (17), when they analyzed the incubation period to discriminate the reaction of three FHIA genotypes to Yellow and Black Sigatoka under natural infection. The incubation period was also used by Alvarez et al. (18) in the successful evaluation of resistance to Black Sigatoka of plantain and banana genotypes under greenhouse conditions.
Similarly, Abadie et al. (9) demonstrated that incubation period also varied significantly in genotypes with different level of partial resistance in field and in detached leaf assays. Pondering the literature quoted, the incubation period can be consider useful for the early screening of Musa germplasm using mycelial suspensions as infective inoculums under controlled conditions; this variable is also very easy to evaluate, and it is not time consuming.
In our experimental condition, it was used a reproductive protocol that described aspects related to the cultural, morphological, molecular, and aggressiveness characteristics of M. fijiensis strains. It also indicated how to use epidemiological variables and components of resistance to characterize the infective cycle of M. fijiensis in greenhouse. These reasons guaranteed incubation period to be useful for discriminating the response of Musa genotypes.
Number of necrotic lesions and area of necrotic lesions (NNL and ANL)
Mature necrotic lesions were observed only in Grande naine, Pisang Awak and Pisang lilin. No necrosis symptoms were observed in the rest of the genotypes until the final evaluation. Significantly greater were the numbers of necrotic lesions observed in Grande naine and Pisang Awak compared with those in Pisang lilin. The biggest areas of necrotic lesions were measured in Grande naine with significant differences with Pisang Awak and Pisang lilin. The size of necrotic lesions in Pisang Awak was also bigger compared with those in Pisang lilin (Table 3).
Differences regarding the formation of mature necrotic lesions were observed only in the genotypes Grande naine, Pisang Awak and Pisang lilin. This fact indicated that the number and area of necrotic lesions were good quantitative variables to differentiate inoculated Musa genotypes with a variable level of resistance to M. fijiensis in an early stage. This finding reinforced the results of Abadie et al. (9), where the resistant varieties Zebrina and Pisang ceylan had lesser and smaller necrotic lesions and more reduced necrotic areas than other resistant genotypes in the field and in detached leaf assays.
The use of the number and area of necrotic lesions in M. fijiensis to evaluate the epidemiology and ecology of Black Sigatoka on plantain and banana (Musa spp.) in Costa Rica under field condition was first reported by Gauhl (13). However, counting of total necrotic lesions on inoculated Musa leaves is a laborious, time consuming and tedious task because of the great number of lesions and coalescences that may be observed. Nevertheless, the number and area of necrotic lesions may also contribute in studies related to M. fijiensis aggressiveness variability, the efficacy of fungicide protection, and the infective capacity of different inoculants.
The number of necrotic lesions was used successfully by Seifbarghi et al. (19) to determine the host range of Septoria species in inoculation experiments under controlled conditions of 27 genotypes of some cereals and wild grasses. Suffert et al. (20), by using the area of necrotic lesions, provided a good measure of M. graminicola fitness in estimating the quantitative resistance of wheat to Mycosphaerella graminicola blotch and characterizing the differences among isolates within a pathogen population.
Infection index (II)
The infection index was calculated only in Grande naine, Pisang Awak and Pisang lilin which showed mature necrotic lesions at 63 DPI. Statically significant differences were determined among the three genotypes, where Grande naine reached the highest percentage (87.03) of leaflet area with necrotic tissue, followed by Pisang Awak (61.28) and Pisang lilin (44.61).
Severity or Infection intensity caused by Mycosphaerella spp. has been used in the evaluation of Musa spp. response under natural infection conditions (21). However, other authors, instead of using diagrammatic scales, have calculated infection intensity according to the symptoms described by Meredith and Lawrence (22) and Fouré (10). Gauhl (13) determined the infection index by using the function proposed by Lehmann Danziger (21) and carried out an epidemiological and ecological study on Black Sigatoka disease in plantain and banana in Costa Rica where the response among the natural infected genotypes were statistically different. Orjeda (23) provides details on Musa spp. evaluation trials carried out in nine sites worldwide in which the infection index was successfully used to determine the response of the genotypes evaluated to Black Sigatoka.
Likewise, Carlier et al. (24) confirmed the usefulness of the infection index to follow up Mycosphaerella spp leaf spot diseases throughout different phenological development stages (six months, flowering, harvest) in Musa germplasm. Krishnamoorthy et al. (25) differentiated the response of 11 banana hybrids and their respective parents after natural infection of M. fijiensis in field trials. Rocha et al. (26) used the infection index to develop a temporal analysis to assess the aerobiology of Mycosphaerella musicola spores and determine the evolution of the disease progression curve.
The infection index was successfully used by Portal et al. (27) to characterize Mycosphaerella fijiensis mutants transformed with a green fluorescent protein-carrying construct by using a restriction enzyme-mediated integration methodology. These authors observed that GFP-18 transformant showed increased aggressiveness in susceptible Grande naine and resistant Yangambi km5 plants once infection index was evaluated in a greenhouse.
Finally, the infection index depends on the level of resistance of the inoculated genotypes, and in the present study, it was calculated only in Grande naine, Pisang Awak and Pisang lilin, where necrotic tissues were observed.
Area under disease-progress curve (AUDPC)
The AUDPC values calculated from the data in all the evaluations until day 56 showed statistically significant differences among the inoculated genotypes respect to their quantitative disease resistance. The greatest AUDPC values were reached by Grande naine and Pisang Awak, while the lowest values were calculated in FHIA-18 and FHIA-25. However, Pisang lilin, Yangambi Km 5 and Calcutta 4 revealed intermediate values.
This paper shows the first implementation of the AUDPC for the quantitative assessment of the disease resistance of Musa genotypes artificially inoculated with the causal agent of Black Sigatoka in greenhouse. Jeger et al. (28) used AUDPC to evaluate disease resistance in different crop cultivars, and they concluded that this variable was useful to measure the quantitative disease resistance in repeated assessments of the disease. Commonly, AUDPC may be practical for investigating the effectiveness of fungicide applications to control Mycosphaerella leaf pathogen diseases in field condition (29) and the evaluation of plant disease resistance (30).
Kablan et al. (30) showed that the AUDPCs calculated in banana plants grown with silicon were significantly lesser than those calculated for plants with no silicon. Thanks to the use of this quantitative variable, they integrated pest management against M. fijiensis by reducing the disease pressure on banana.
Otherwise, Cuéllar et al. (31) determined that the AUDPC and the apparent infection rate (r) were the only useful quantitative variables to differentiate resistance of plantain and banana genotypes and aggresiveness of Black Sigatoka strains during Musa-M. fijiensis interaction under controlled condition.
Significant differences were obtained among the inoculated Musa genotypes, and it may be the starting point for Musa breeders and epidemiologists to develop descriptive and more precise growth model for the early screening of BLSD that could be used in identifying resistant genotypes to M. fijiensis.
Asexual latent period (ALP)
Asexual latent period was detected only in Grande naine, Pisang Awak and Pisang lilin genotypes. Grande naine (41,22 days) and Pisang Awak (44,72 days) had statistically significant shorter (approximately 14 days) ALP than Pisang lilin (56,50 days).
The asexual latent period clearly differentiated the response of Grande naine, Pisang Awak and Pisang lilin from the rest of the inoculated genotypes. It was demonstrated experimentally for the first time that M. fijiensis conidia could be obtained with inoculation assays using mycelial fragments in greenhouse conditions. Indeed, some Musa spp. were able to complete the asexual cycle within the evaluated period (63 DPI), and there was correspondence between the asexual latent period and the level of resistance among the genotypes. Browne et al. (32) used the ALP as a component of partial disease resistance in wheat, detected in a detached leaf assay with the inoculation of Microdochium majus, and where cultivar responses were possible to be differentiated. Nevertheless, Dita et al. (33) evaluated the ALP and the spore production in four potato genotypes artificially inoculated with Alternaria solani Sorauer, but the separation of cultivars according to resistance levels could not be obtained. Similarly, no difference was found in the number of asexual spores observed on seven partially resistant and susceptible Musa cultivars in the field and ten cultivars under controlled conditions with detached leaf assays (9).
Suffert et al. (20) identified ALP, development rate of sporulating area, maximal sporulating area, pycnidial density, and sporulation capacity traits as the most relevant variables to describe aggressiveness in Mycosphaerella graminicola (Septoria tritici blotch) population. They suggested that these variables could be used to estimate the quantitative resistance of wheat to this fungal pathogen.
Further studies should be led to improve the control of experimental conditions, the quantification methodology, and the inclusion of new Musa genotypes.
Number of spermogonia
The greatest number of spermogonia was observed in Grande naine and Pisang Awak, followed by Pisang lilin. Few spermogonia were detected in Calcutta 4 and Yangambi Km 5 and the lowest quantities were counted in FHIA-18 and FHIA-25 (Table 4). Spermogonia were only detected in Grande naine, Pisang Awak and, in a significant less number, in Pisang lilin (Table 4). The rest of the genotypes did not produced conidia.
In this work, the number of spermogonia was used as a component of resistance for the early evaluation of Musa genotypes artificially inoculated with Black Sigatoka in greenhouse. Significant statistically differences were obtained among the inoculated genotypes, and it was experimentally demonstrated that spermogonia of M. fijiensis could be obtained by inculating Musa spp. with mycelial fragments in greenhouse assays.
Pérez et al. (34) used the number of spermogonia and pseudothecia for the first time to compare Grande naine and FHIA-18 response to BLSD in two localities of Cuba, and they differentiated the resistance level of both genotypes. Nevertheless, this variable has not been used before under controlled conditions for the early discrimination of resistance in Musa spp.
In our experience, it was impossible to obtain pseudothecia of M. fijiensis because the inoculation assay was performed with only one isolate (CCIBP-Pf80), and the heterothaly nature of M. fijiensis is well known. The sexual cycle of M. fijensis could be made possible under controlled conditions by inducing pseudothecia in plants artificially inoculated with isolates of different mating types in a greenhouse. The sexual latency period and the number of ascospores could then be calculated to be used as other components of resistance in Musa breeding programs.
CONCLUSIONS
Results indicated that resistance to Black Sigatoka in artificially inoculated genotypes could be evaluated by components of resistance to assess the response of Musa spp. to Mycosphaerella fijiensis Morelet quantitatively, which would make more efficient and precise the early evaluation process to support banana and plantain breeding programs. Further genotypes with known resistance, partial resistance and susceptible phenotypes in field conditions must be confirmed in subsequent greenhouse tests. These results may be useful for screening BLSD Musa resistant breeding material, evaluation of aggressiveness of M. fijiensis isolates, studies related to molecular plant-pathogen interaction, and management schemes of BLSD. Additionally, these findings will supply information for guiding future studies on mechanisms involved in the BLSD resistance in Musa spp by dissecting the infection cycle of M. fijiensis under controlled condition.
ACKNOWLEDGEMENTS
This research was supported by Instituto de Biotecnología de las Plantas, Universidad Central de las Villas, Villa Clara, Cuba. We are also grateful to the International Foundation for Science for the financial support to this investigation in the framework of the IFS Research Grant Agreement NO.C/4296-1. We are really gratefull to DrC. Eduardo Sistachs for his contribution with the editing process and English revision.
REFERENCES
1. Churchill CLA. Mycosphaerella fijiensis, the black leaf streak pathogen of banana: progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Molecular Plant Pathology. 2011;12(4):307-328.
2. Cavalcante MJB, Escalante J, Madeira JP, Romero RE, Nicole RM, Oliveira CL, et al. Reactive oxygen species and cellular interactions between M. fijiensis and banana. Tropical Plant Biol. 2011;4:134-143.
3. Castelan FP, Abadie C, Hubert O, Chilin-Charles Y, de Lapeyre de Bellaire L, Chillet M. Relation between the severity of Sigatoka disease and banana quality characterized by pomological traits and fruit green life. Crop Protection. 2013;50:61-65.
4. Pillary M. Classical genetics and traditional breeding in Musa. In: Genetic, genomics and breeding of bananas. Editors (Michael Pillary, George Ude, Chittaranjan Kole). 2012; Pp 34-55.
5. Ortiz R. Conventional banana and plantain breeding. Acta Hort. (ISHS). 2013;986:177-194.
6. Robert S, Ravigne V, Zapater MF, Abadie C, Carlier J. Contrasting introduction scenarios among continents in the worldwide invasion of the banana fungal pathogen Mycosphaerella fijiensis. Molecular Ecology. 2012;21(5):1098-1114.
7. Amorim EP, Santos-Serejo D, Amorim VBO, Ferreira CF, Silva SO. Banana breeding at EMBRAPA cassava and fruits. Acta Hort. (ISHS). 2013;986:171-176.
8. Mourichon X, Peter D, Zapater M. Inoculation expérimentale de Mycosphaerella fijiensis Morelet, sur jeunes plantules de bananier issues de culture in vitro. Fruit. 1987;42(4):195-198.
9. Abadie C, Elhadrami A, Leiva-Mora E, Carlier J. Efficiency and durability of partial resistance against black leaf streak disease. In: Mycosphaerella leaf spot disease of bananas, present status and outlook. Proceding of the Workshop on Mycosphaerella leaf spot disease, San José, Costa Rica, INIBAP. 2003.
10.Fouré E. Les cercosporioses du bananier et leurs traitements: Etude de la sensibilité variétale des bananiers et des plantains á Mycosphaerella fijiensis Morelet au Gabon. Fruit. 1982;37:749-771.
11.Leiva-Mora M, Alvarado-Capó Yelenys, Acosta-Suárez Mayra, Cruz-Martín Mileidy, Sánchez García Cynthia, Roque Berkis. Protocolo para la inoculación artificial de M. fijiensis y evaluación de la respuesta de Musa spp. con variables epifitiológicas y componentes de la resistencia. Biotecnología Vegetal. 2010;10(2):79-88.
12.Stover RH. Distribution and cultural characteristics of the pathogens causing banana leaf spot. Tropical Agriculture Trinidad. 1976;53:111-114.
13.Gauhl F. Epidemiology and Ecology of black Sigatoka (Mycosphaerella fijiensis Morelet) on Plantain and Banana (Musa spp.) in Costa Rica, Central America. Montpellier, Francia, INIBAP. 1994.
14.Shaner G, Finney RE. The effect of Nitrogen fertilization on the expression of show-mildewing resistance in Knox wheat. Phytopathology. 1977;67:1051-1056.
15.Alvarado Y, Leiva M, Rodríguez MA, Acosta M, Cruz M, Portal N, et al. Early evaluation of Black leaf streak resistance by using mycelial suspension of Mycosphaerella fijiensis. En: Jacome L, Leproive P, Martin D, Ortiz R, Romero R, Escalant JV (eds). Mycosphaerella leaf spot diseases of bananas: present status and outlook. 2003; 169-175. INIBAP, Montpellier. ISBN 2910810-57-7.
16.Aguirre-Gaviria MC, Castaño-Zapata J, Zuluaga-Arias LE. Rapid diagnostic method of Mycosphaerella musicola Leach and Mycosphaerella fijiensis Morelet, causal agents of Yellow Sigatoka and Black Sigatoka. INFOMUSA. 1999;8(2):7-9.
17.Molina-Tirado OI, Castaño-Zapata J. Análisis de algunos componentes de la resistencia en los híbridos de banano y plátanos FHIA 01, FHIA 17 y FHIA 21 a la Sigatoka Negra (Mycosphaerella fijiensis Morelet) y amarilla (Mycosphaerella musicola Leach). Rev Acad Coloma Cienc. 2003;27(103):181-190.
18.Alvarez E, Cuellar A. Evaluating the resistance of plantain and banana genotypes to Black Sigatoka (Mycosphaerella fijiensis) under greenhouse conditions. 2010; American Phytopathology Society Annual Meeting. Celebrated in August 7-11. available in http://www.apsnet.org/meetings/Documents/2010_Meeting_Abstracts /a10ma27.htm.
19.Seifbarghi S, Razavi M, Aminian H, Zare R, Etebarian H. Studies on the host range of Septoria species on cereals and some wild grasses in Iran. Phytopathologia Mediterranea. 2009;48(3):422-429.
20.Suffert F, Sache I, Lannou C. Assessment of quantitative traits of aggressiveness in Mycosphaerella graminicola on adult wheat plants. Plant pathology. 2013.
21.Lehmann-Danziger H. Ausbreitung, Bewertung und Strategien zur Bekampfung der Schwarzen Sigatoka-Krankheit: Untersuchungen an Kochbananen in Mittel- und Sudamerika. Der Tropenlandwirt Beiheft. 1988;34:133-177.
22.Meredith DS, Lawrence JS. Black leaf streak disease of bananas (Mycosphaerella fijiensis): Symptoms of disease in Hawaii, and notes on the conidial state of the causal fungus. Transaction British Mycology Society. 1969;52:459-476.
23.Orjeda G. Evaluating bananas: a global partnership. Results of IMTP Phase II (G. Orjeda ed). International Network for the Improvement of banana and plantain, Montpellier, Francia. 2000.
24.Carlier J, Waele D, Escalant JV. Evaluación global de la resistencia de los bananos al marchitamiento por Fusarium, enfermedades de las manchas foliares causadas por Mycosphaerella y nematodos. Evaluación del comportamiento. (A. Vézima y C. Picq, eds). Guias técnicas INIBAP 6. Red para el mejoramiento del banano y el plátano, Montpellier, Francia. 2002.
25.Krishnamoorthy V, Kumar N, Angappan K, Soorianathasundaram K. Evaluation of new banana hybrids against black leaf streak disease. InfoMusa. 2004;13(1):25-27.
26.Rocha SH, Pozza AE, Uchôa DNC, Cordeiro ZJM, De Souza EP, et al. Temporal Progress of Yellow Sigatoka and Aerobiology of Mycosphaerella musicola Spores. Jour Phytopathol. 2012;160(6):277-285.
27.Portal O, Acosta-Suárez M, Ocaña B, Schäfer W, Jiménez E, Höfte M. A green fluorescent protein transformed Mycosphaerella fijiensis strain shows increased aggressiveness on banana. Australasian Plant Pathology. 2012;41(6):645-647.
28.Jeger MJ, Viljanen-Rollinson SLH. The use of the area under the disease-progress curve (AUDPC) to assess quantitative disease resistance in crop cultivars. Theoretical and Applied Genetics. 2001;102(1):32-40.
29.Burke JJ, Dunne B. Investigating the effectiveness of the Thies Clima «Septoria Timer» to schedule fungicide applications to control Mycosphaerella graminicola on winter wheat in Ireland. Crop protection. 2008;27(3):710-718.
30.Kablan L, Lagauche AE, Delvaux BE, Legrève A. Silicon Reduces Black Sigatoka Development in Banana. Plant Diseases. 2012;96(2):273-278.
31.Cuéllar QA, Álvarez CE, Castaño ZJ. Evaluación de Resistencia de Genotipos de Plátano y Banano a la Sigatoka Negra (Mycosphaerella fijiensis Morelet). Rev Fac Nal Agr Medellín. 2011;64(1):5853-5865.
32.Browne RA, Mascher F, Golebiowska G, Hofgaard IS. Components of Partial Disease Resistance in Wheat detected in a detached leaf assay inoculated with Microdochium majus using First, Second and Third Expanding Seedling Leaves. Journal of Phytopathology. 2006;154(4):204-208.
33.Dita RMA, Brommonschenkel SH, Matsuoka K, Mizubuti ESG. Components of Resistance to Early Blight in Four Potato Cultivars: Effect of Leaf Position. Jour Phytopathol. 2006;154:230-235.
34.Pérez-Miranda M, Pérez VL, Trujillo R, Betancourt DM. Variability of Mycosphaerella fijiensis Morelet. Durability of the Resistance to Black Sigatoka in the FHIA hybrids cultivars. FITOSANIDAD. 2006;10(1):37-47.
Recibido: 24-11-2013.
Aceptado: 4-9-2014.
* Correspondence: Michel Leiva-Mora. E-mail: michel@ibp.co.cu.