Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Protección Vegetal
versión impresa ISSN 1010-2752versión On-line ISSN 2224-4697
Rev. Protección Veg. vol.30 no.1 La Habana ene.-abr. 2015
ORIGINAL ARTICLE
Biological control of Polyphagotarsonemus latus (Banks) by the predatory mite Amblyseius largoensis (Muma) on sheltered pepper production in Cuba
Control biológico de Polyphagotarsonemus latus (Banks) con el ácaro depredador Amblyseius largoensis (Muma), en la producción protegida de pimiento en Cuba
Héctor RodríguezI,*, Adrián MontoyaII, Ileana MirandaI, Yaritza RodríguezIII, Tomás L. DepestreIII, Mayra RamosIV, Mohammad H. Badii-ZabehV
IGrupo Plagas Agrícolas, Dirección de Protección de Plantas. Centro Nacional de Sanidad Agropecuaria (CENSA). Carretera de Jamaica y Autopista Nacional. Apdo 10, San José de las Lajas, Mayabeque, CP 32 700, Cuba.
IIFacultad Agroforestal de Montaña (FAM). Centro Universitario de Guantánamo (CUG). El Salvador, Guantánamo, Cuba.
IIIInstituto de Investigaciones Hortícolas Liliana Dimitrova (IIHLD). Carretera Bejucal-Quivicán, km 33½, Quivicán, Mayabeque, Cuba.
IVDepartamento de Medio Ambiente. Facultad de Gestión de la Ciencia, la Tecnología y el Medio Ambiente, Instituto Superior de Tecnologías y Ciencias Aplicadas (InSTEC). Carlos III y Luaces, Plaza de la Revolución, La Habana. Cuba.
VUANL, San Nicolás, N.L., México.
ABSTRACT
The effectiveness of Amblyseius largoensis (Muma) (Acari: Phytoseiidae) for regulating Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae) populations in pepper crops was evaluated in a greenhouse, protected microplots and a tunnel system. A. largoensis were released at the densities of 2, 4 and 8 predators per plant in the greenhouse. In the microplot and tunnel systems, only four predators per plant were released. Broad mite population densities were evaluated weekly by sampling young leaves from the top of the plants. All three released rates of A. largoensis significantly (p < 0.05) controlled broad mite populations in the greenhouse; however, the control effect was delayed when only two predatory mites were released. On the other hand, releasing A. largoensis in the microplots and tunnel was also effective in controlling broad mites. These results demonstrated the effectiveness of A. largoensis to control broad mites in the pepper crop.
Key words: broad mite, Tarsonemidae, Phytoseiidae, Capsicum annuum, release rate.
RESUMEN
En el presente estudio se evaluó la efectividad de Amblyseius largoensis (Muma) (Acari: Phytoseiidae) para regular las poblaciones de Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae) sobre pimiento en casas de malla, canaleta y túnel de cultivo protegido. En casas de malla se usaron tres tasas de liberación, 2, 4 y 8 A. largoensis por plantas, mientras que en canaleta y túnel solo se liberaron 4 depredadores por planta. La población del ácaro blanco se evaluó a través de muestreos semanales de hojas jóvenes de la parte superior de las plantas. Las tres tasas de liberación de A. largoensis redujeron significativamente (P < 0,05) las poblaciones del ácaro blanco en casa de malla; pero cuando solo se liberaron dos depredadores por plantas, el efecto de control se retardó. En canaleta y túnel la liberación de A. largoensis también fue eficaz en el control del ácaro blanco. Los resultados obtenidos demostraron que A. largoensis es un agente de control biológico efectivo del ácaro blanco en pimiento.
Palabras clave: ácaro blanco, Tarsonemidae, Phytoseiidae, Capsicum annuum, tasa de liberación.
INTRODUCTION
The broad mite, Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae), is a serious pest in tropical and subtropical regions and has been collected from about 60 different plant families (1). This mite attacks young growing plant parts and is very small and difficult to be detected. It usually feeds on the lower leaf surface making the leaf edges become rigid and roll under, causes distortion and/or discoloration of the flowers and blistering of the fruits, and finally the reduction of the plant yield. P. latus is an important pest of vegetables, and peppers show a low tolerance level against this pest mite (2,3).
In Cuba, P. latus is a serious pest of the sheltered pepper plants (4). The cultivars used at present are severely damaged. Ten mites on a young pepper plant can cause a significantly lesser leaf area and plant height (5). In Cuba, P. latus is controlled almost exclusively with pesticides; however, the effectiveness declines occasionally (6). Therefore, on the basis of these data, non-chemical control solutions, particularly, environmental friendly biological control method as a viable alternative to control this pest mite is being considered in Cuba.
A number of predators of the broad mite, including phytoseiid mite species, have been reported in the literature (1). Species such as Neoseiulus barkeri (Hughes) (7), N. californicus (McGregor) (8), N. cucumeris (9), and more recently Amblyseius swirskii Athias-Heriot (3,10) were reported to control the broad mite successfully in greenhouses. Among these phytoseiid species, only N. californicus has been reported in Cuba, but it has not been observed in association with P. latus.
Amblyseius largoensis (Muma) (Acari: Phytoseiidae) is a very frequent and abundant phytoseiid mite species in Cuba and has been observed feeding on P. latus. Studies have shown this predatory mite to have a short life cycle, high fecundity rate, and an adequate searching efficiency, with a type II functional response when feeding on P. latus as prey (11,12). Therefore, A. largoensis could be considered a good candidate for the biological control of the broad mite. The aim of this research was to evaluate the efficacy of this predator mite to control broad mites on peppers grown in greenhouse and protected systems.
MATERIAL AND METHODS
All experiments were conducted in a greenhouse of the Centro Nacional de Sanidad Agropecuaria (CENSA) located in San José de las Lajas municipality, with net walls and plexiglass roofs and also in areas of protected crop production at the Instituto de Investigaciones Hortícolas «Liliana Dimitrova» (IIHLD) located in Quivicán municipality, both in Mayabeque Province.
The sweet pepper seedlings used for the experiments were obtained under controlled conditions at the Acarology Laboratory (CENSA). One month after germination, the seedlings were transplanted to 5 L plastic pots containing Compacted Red Ferralitic soil (13) and organic matter (cattle manure) in a 3:1 ratio (greenhouse trial) or to the microplots and the tunnels (field trials). The plants were watered according to standard agricultural practices for the sheltered pepper production (14).
Rearing of P. latus and A. largoensis: The broad mite colony used to inoculate pepper plants was maintained according to the detached leaf culture method on pepper leaf (Capsicum annuum L. cv. LPD-5 F1). The predatory mite A. largoensis was mass reared using the tray method with the bean (Phaseolus vulgaris L. var. Fósforo-40) as host plant and Tetranychus tumidus Banks (Acari: Tetranychidae) as prey species (15), both under laboratory conditions. The temperature and the relative humidity, measured with a digital thermo-hygrometer (Testo 608-H2), were of 26.63 ± 6.5°C and 64.08 ± 5.1%, respectively.
Trial in greenhouse: Twenty one days after the transplantation, each pepper plant was inoculated with five females of P. latus. On days 7, 10 and 15 afterwards, two, four or eight females of A. largoensis were released per plant, respectively, no-predator (absolute control) and dicofol-sprayed (positive control) (dicofol CE 18.5 at 0.27 kg ia.ha-1) were also set up. Each release rate was tested independently with their respective controls. There were 12 plants per plastic pot for each treatment. These were placed in three different sites and repeated three times per site. The temperature and the relative humidity, measured with a digital thermo-hygrometer (Testo 608-H2), were of 30.12±2.25ºC and 65.35±3.61%, respectively.
The broad mite populations were examined before the introduction of A. largoensis and also prior to the application of dicofol. After the infestation, plants were sampled weekly for six consecutive weeks. At each sampling, the number of all active stages of broad mites on one leaf per plant collected from the apical zone of each plant was determined under a Zeiss Stemi SV-6 stereomicroscope at 25x.
Trial in protected microplots: In a 10 x 5 m tunnel covered with plastic, five microplots were planted each with two rows of 20 pepper plants. Thirty days after the transplantation, every pepper plant was inoculated with five females of P. latus. During the 5-7 following days, four females of the predatory mite were released on each plant, in the left-hand side of the microplot; the central part of the microplot was used as no-release no-predator control plot, and the right-hand side of the microplot was chosen as the control plot with Dicofol (dicofol CE 18.5 at 0.27 kg ia.ha-1). The temperature and the relative humidity, measured with a digital thermo-hygrometer (Testo 608-H2), were of 32.46±2.21ºC and 73.84±5.25%, respectively.
The broad mite populations were examined before the introduction of A. largoensis and also prior to the application of dicofol. After the infestation, the plants were sampled weekly for six consecutive weeks. In each sampling, the number of broad mites (all active phases) on one leaf collected from the apical zone of fifteen randomly selected plants was determined under a Zeiss Stemi SV-6 stereomicroscope at 25x. In this trial, each plant was considered a replicate.
Trial in tunnel: In a 20 x 10 m tunnel covered with plastic, six rows were planted with 60 pepper plants each. Thirty days after the transplantation, every pepper plant was inoculated with five females of P. latus. On the 5-7 following days and also four week later, the predatory mite was released. The tunnel was transversely divided into three sections by means of a double layer of plastic wall. Each section housed approximately 60 plants. In each section, a treatment was set up; in the southern section, four immature phases of A. largoensis were released per plant; the central section was used for the no-predator control and the northern section for the dicofol-sprayed control (dicofol CE 18.5 at 0.27 kg ia.ha-1). Temperature was measured with a thermometer. The mean temperature was of 35.11± 6.02ºC, with average maxima and minima of 41.04±2.52 and 22.33±2.98ºC, respectively,
The broad mite populations were examined before the introduction of A. largoensis and also prior to the application of dicofol. After the infestation, the plants were sampled weekly for six consecutive weeks. In each sampling, the number of broad mites (all active phases) on one leaf collected from the apical zone of twenty randomly selected plants was determined under a Zeiss Stemi SV-6 stereomicroscope at 25x. In this trial, each plant was considered a replicate.
In all experiments conducted, A. largoensis populations were examined weekly after plant infestation. In each sampling, the number of predatory mites present on one leaf collected from the apical zone and also on an additional leaf from the middle zone of each plant was determined under a Zeiss Stemi SV-6 stereomicroscope at 25x.
Statistical analysis: Prior to ANOVA running, the homogeneity of the variances was tested. Data were normalized using Öx+0.5 transformations followed by ANOVA analysis using SAS version 9.0 software (SAS Institute, Inc, 2002). The means were compared by Tukey HSD at a = 0.05. Data points show the means ± SEM.
RESULTS
Trial in greenhouse
The results of the release of A. largoensis for controlling broad mites on peppers in greenhouse are shown in Figure 1. The dicofol treatment always caused a rapid decline in broad mite population; however, the broad mite density tended to increase again at the end of the trials. In the treatment in which A. largoensis was released at two individual mites per plant (Fig. 1A), the number of broad mite continued increasing for two consecutive weeks; however, by the third week, there was a reduction in the number of broad mites. This trend continued until the end of the trial. Nevertheless, from the second sample, the treatment with A. largoensis reduced the broad mite density significantly to the end of the trial (P < 0.001, F = 29.55, df = 2) when compared with the no-predator control. The density of broad mites in the control treatment peaked in the second sampling and it gradually declined thereafter.
When four predatory mites were released, (Fig. 1B), the broad mite density continued increasing for one more week, and thereafter, it started to decline until the end of the trial. Seven days after the predator release, the treatment with A. largoensis reduced the broad mite density significantly (P < 0.0001, F = 133.7, df = 2) to the fourth sampling when compared with the no-predator control. During the fifth and sixth samplings, no significant difference between broad mite density in A. largoensis treatment and the control was observed. The density of broad mites in the control showed a similar behavior as in the previous trial.
When eight predatory mites per plant were released, (Fig. 1C), the broad mite density showed a similar dynamic pattern as in the second trial; however, with the exception of the third (P < 0.002, F = 7.80, df = 2) and sixth (P < 0.0001, F = 181.14, df = 2) samplings, the reduction of the broad mite density by A. largoensis was not significant as compared with the control treatment.
Trial in protected microplots
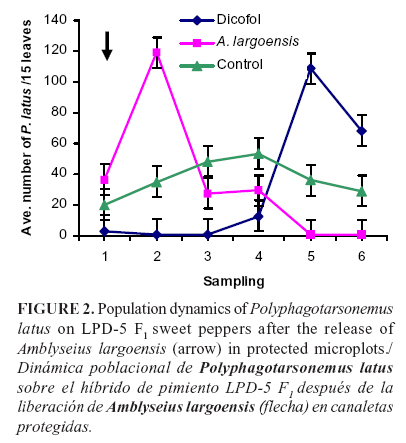
The results of releasing A. largoensis on broad mite population in covered microplots are shown in Figure 2. In the first sampling after the release of the predatory mite, the broad mite population continued increasing; however, this trend was reversed in the following samplings and toward the end of the trial. Finally, the reduction in the broad mite density by the predator was significant (P < 0.05, F = 3.33, df = 2) from the fourth sampling onward.
Trial in tunnel
In the tunnel system, the results were similar to those obtained in the protected microplots (Fig. 3). In the first sampling after the release of the predatory mite, the broad mite population continued increasing; however, as in the previous case, this trend was reversed after the second sampling toward the end of the trial. The density of broad mites in the control treatment peaked in the second sampling and it gradually declined thereafter.
The predatory mite individuals were found primarily on leaves located at the middle of the pepper plants. The population means oscillated with an average of 2 to 6 predatory mites per sampling (Fig. 4), with a further increase at the end of the experiments.
DISCUSSION
The production of crops under protected conditions such as climatically controlled greenhouses and glasshouses, protected areas covered by plastic sheets, or «insect-proof screening» (tunnels) with little or no climate control is increasing worldwide (16). The agronomic and production conditions of the crops in these systems are also suitable for the development of new pests and the population rise of other species to levels that may be incompatible with the obtaining of acceptable yields. In this context, an increase of the economic impacts by nematodes, insects and mites has occurred with the latter species causing considerable losses in some horticultural species grown under these protected conditions (17).
The present study showed that A. largoensis was an effective predator on P. latus. In all the greenhouse treatments (2, 4, and 8 predatory mites/plants), an excellent control of the pest by this predatory species was obtained. A gradual reduction of broad mite population was observed over a period of seven weeks.
Regardless of the extremely high density of the broad mite, the most favorable result was obtained when eight A. largoensis/plant were released. With 2 and 4 predatory mites/plant, the control effect was observed a week later as compared with the 8 predators/plant; however, the results were the same at the end of the trials.
Our present findings are in agreement with previous studies on the biological characteristics of A. largoensis, whereby it was shown that this predaceous mite had a potential to be a highly effective predator of the broad mite (11,12). It is important to consider that the release rate used in this study was much lower than the commonly used release rates for other phytoseiid mites (3,9,18).
Obtaining an effective broad mite control in microplots and tunnel system required a slightly longer time period as compared with the greenhouse system, but with similar results at the end of the trials. It should be mentioned that in microplots and tunnel condition, the systems are more open and the temperature is extremely high, and therefore, the environment is more favorable for the development of P. latus (1).
Amblyseius largoensis was mainly localized at the middle of the plant. A very high temperature and/or an intense light at the top of the pepper plants during the day could cause the movement of the predator towards the inside foliage of the pepper plants. Weintraub et al. (9) considered that N. cucumeris moved upwards and downwards on the plant as the light intensity and temperature changed throughout the day.
In Cuba for several years, A. largoensis has been evaluated as a promising candidate for the biological control of P. latus. Our results showed that it has proved to be an effective control agent for the broad mite with an excellent establishment rate in sheltered peppers systems. In an experiment conducted under controlled conditions, A. largoensis was compatible with the miticides dicofol, sulphur and the LBt-13 strain of Bacillus thuringiensis Berliner, the insecticide imidacloprid, and the fungicides mancozeb and metalaxyl+mancozeb. However, the insecticides diafentiuron and cypermethrin+diazinon were toxic to the predator (19). These results permit to think that an Integrated Pest Management program (IPM) can be a viable alternative including use of chemicals, host plant resistance and biological control, as it has being suggested by different researchers (20,21).
ACKNOWLEDGEMENTS
We thank Eduardo Sistachs Ph. D. for improving the English text; Francisco J. Ferragut Ph. D. for his comments on the preliminary draft and Reynaldo Chico for his technical support.
REFERENCES
1. Gerson U. Biology and control of the broad mite, Polyphagotarsonemus latus (Banks)(Acari: Tarsonemidae). Exp Appl Acarol. 1992;13:163-178.
2. De Coss-Romero M, Peña JE. Relationship of broad mite (Acari: Tarsonemidae) to host phenology and injury levels in Capsicum annuum. Fla Entomol. 1998;81:515-526.
3. Tal C, Coll M, Weintraub PG. Biological control of Polyphagotarsonemus latus (Acari: Tarsonemidae) by the predaceous mite Amblyseius swirskii (Acari: Phytoseiidae). IOBC/WPRS Bull. 2007;30:25-36.
4. Montoya A, Miranda I, Rodríguez Y, Rodríguez H. Percepción de los fitosanitarios sobre el control de Polyphagotarsonemus latus en la producción protegida de pimiento. Rev Protección Veg. 2013;28(1):60-64.
5. Rodríguez H, Montoya A, Miranda I, Rodríguez Y, Ramos M. Influence of the phenological phase of two pepper cultivars on the behaviour of Polyphagotarsonemus latus (Banks). Rev Protección Veg. 2011;26(2):73-79.
6. Montoya A, Miranda I, Rodríguez Y, Ramos M, Rodríguez H. Incidencia de Polyphagotarsonemus latus (Banks) en la producción protegida de pimiento (Capsicum annuum L. var. Lical). Centro Agrícola. 2012;39(1):53-58.
7. Fan Y, Petitt FL. Biological control of broad mite, Polyphagotarsonemus latus (Banks), by Neoseiulus barkeri Hughes on pepper. Biol Control. 1994;4:390-395.
8. Peña JE, Obsorne L. Biological control of Polyphagotarsonemus latus (Banks) (Acarina: Tarsonemidae) in greenhouses and field trials using introduction of predacious mites (Acarina: Phytoseiidae). Entomophaga. 1996;41:279-285.
9. Weintraub PG, Kleitman S, Mori R, Shapira N, Palevsky E. Control of broad mites (Polyphagotarsonemus latus (Banks)) on organic greenhouse sweet peppers (Capsicum annuum L.) with the predatory mite, Neoseiulus cucumeris (Oudemans). Biol Control. 2003;26:300-309.
10.Van Maanen E, Vila E, Sabelis MW, Janssen A. Biological control of broad mites (Polyphagotarsonemus latus) with the generalist predator Amblyseius swirskii. Exp Appl Acarol. 2010;52:29-34.
11.Rodríguez H, Miranda I, Ramos M, Badii MH. Functional and numerical responses of Amblyseius largoensis (Muma) (Acari: Phytoseiidae) on Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae) in Cuba. Internat J Acarol. 2010;36:371-376.
12.Rodríguez H, Ramos M, Montoya A, Rodríguez Y, Chico R, et al. Development of Amblyseius largoensis as biological control agent of the broad mites (Polyphagotarsonemus latus). Biotecnología Aplicada. 2011;28(3):171-175.
13.MINAGRI. Ministerio de la Agricultura. Instituto de Suelos. Nueva versión de la clasificación genética de los suelos de Cuba. La Habana. AGRINFOR, 1999. 64 p.
14.Casanova AS, Gómez O, Hernández M, Chailloux M, Depestre T, Pupo FR. Instituto de Investigaciones Hortícolas Liliana Dimitrova, Ministerio de la Agricultura. Manual para la Producción Protegida de Hortalizas. 2da Edición. Editorial Liliana, 2007, 179 pp.
15.Montoya A, Miranda I, Ramos M, Rodríguez H. Cría de Amblyseius largoensis (Muma) sobre Tetranychus tumidus (Banks) utilizando el método de las bandejas. Rev Protección Veg. 2009;24:191-194.
16.Gerson U, Weintraub PG. Mites for the control of pests in protected cultivation. Pest Manag Sci. 2007;63:658-676.
17.Hanafi A. Invasive pests and diseases: a challenge to IPM in greenhouse crops. Phytoparasitica. 2005;33:423-426.
18.Weintraub PG, Alchanatis V, Palevsky E. Distribution of the predatory mite, Neoseiulus cucumeris, in greenhouse pepper. In: Cantliffle DJ, Stoffella PJ, Shaw N (editors). Proc. VIII Symposia on Prot. Cult. Mild Winter Climates. Acta Hort. 659, ISHS 2004.
19.Montoya A, Pino O, Rodríguez H, Posos P. Selectividad de Amblyseius largoensis (Muma) a productos fitosanitarios en la producción protegida de pimiento (Capsicum annuum L.). Rev Protección Veg. 2013;28(1):65-69.
20.Martin T, Assogba-Komlan A, Sidick I, Ahle V, Chandre F. An acaricide-treated net to control phytophagous mites. Crop Protection. 2010;29:470-475.
21.Angeli LF, de Queiroz DL, Piacentini D. Damage characterization and control tactics to broad mite (Polyphagotarsonemus latus Banks) in Paraguay-tea plants (Ilex paraguariensis A.St.-Hil.). R Bras Bioci., Porto Alegre. 2010;8(2):208-212.
Recibido: 2-7-2014.
Aceptado: 10-10-2014.
*Dirección actual: Departamento Biología-Sanidad Vegetal. Facultad de Agronomía. Universidad Agraria de La Habana (UNAH). Carretera de Tapaste y Autopista Nacional. San José de las Lajas, Mayabeque. CP 32 700. Cuba. E-mail de contacto: morell_66@unah.edu.cu.