Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Vaccimonitor
versión impresa ISSN 1025-028Xversión On-line ISSN 1025-0298
Vaccimonitor vol.24 no.3 Ciudad de la Habana sep.-dic. 2015
ARTÍCULO ORIGINAL
Development of Novel Protocol for Preclinical Monitoring the Release of Adjuvants Encapsulated Mucosal Delivery Carriers
Desarrollo de un novedoso protocolo para el monitoreo preclínico de sistemas de liberación mucosal con capacidad adyuvante
Mohamed Ibrahim-Saeed,1* Abd Rahaman-Omar,2 Mohd Zobir-Hussein3, Isam Mohamed-Elkhidir4, Samer Hussein-Al-Ali3, Mothanna Sadiq-Al-Qubaisi2, Zamberi Sekawi1
1 University of Putra Malaysia, Medical Faculty, Microbiology & Parasitology dept., Serdang, 34300, Malaysia.
2 University of Putra Malaysia, Institute of Biosciences, Serdang, 34300, Malaysia.
3 University of Putra Malaysia, Institute of Advanced Technology Serdang, 34300, Malaysia.
4 University of Khartoum, Faculty of Medicine, Microbiology & Parasitology dept., Sudan, 11115.
email: zamberi@upm.edu.my
*Virology, Microbiology & Parasitology dept., Medical Faculty, University of Putra Malaysia.
ABSTRACT
This work contributes in vaccines down-stream process by introducing a novel platform for in-vitro monitoring of vaccine-adjuvant delivery profile as a crucial preclinical optimizing step in mucosal vaccines. Nano and micro particles of Calcium phosphate (Cap) vaccine-adjuvant were encapsulated in Chitosan and Alginate polymeric carriers. Adjuvants release profiles monitored in a permeable bag at 37°C, pH 2, incubated in isotonic buffer for 96 hours. The released Calcium in the outer buffer was monitored and compared in-addition to the carrier’s swelling and biophysical properties. The adjuvants and carriers did not interfere with the proliferation of cultured hepatocytes an indicator of their safe use; Chitosan’s viscosity and swelling were higher than Alginate. Chitosan’s Zeta-potential was significantly high positive, while Cap and Alginate were negative. The prepared CaP and Chitosan particles were in nano-size, while the ready-made CaP adjuvant and Alginate were in micro-size using zeta-seizer and scanning electron-micrograph. The release of nano-size particle was in ascending, extended and controlled manner compared to micro-size adjuvant. Moreover, nano-adjuvant release profile from Chitosan was superior compared to Alginate. The core controlling factors in vaccine-adjuvant sustained release includes; smaller adjuvant particles (nano-size), carrier’s low swelling, high viscosity and importantly carrier-adjuvant entrapment reversibility. Chitosan offers sustained ascending superior capacity in releasing Nano-Cap adjuvant. This novel in-vitro pre-clinical study answer a crucial downstream preparative step for optimizing mucosal vaccines before their direct routine in-vivo trial on animal regardless of adjuvant’s particle size or delivery kinetics.
Keywords: nano-adjuvant, delivery carriers, mucosal vaccines.
RESUMEN
Este trabajo contribuye a la investigación de vacunas, a través de una plataforma in vitro que monitorea los perfiles de liberación vacuna-adyuvante, como paso crucial para el desarrollo preclínico de vacunas mucosales. Las nano y macropartículas de fosfato de calcio (Cap), se encapsularon en sistemas de liberación de quitosana y alginato. El perfil de liberación del adyuvante fue monitoreado en membranas permeables a 37ºC, pH2 e incubado en tampón isotónico por 96 horas. Se monitoreó el calcio liberado en el tampón externo y se comparó con la capacidad de hidratación del sistema de liberación utilizado y sus características biofísicas. Los adyuvantes y sistemas de liberación no interfirieron con la proliferación de cultivos de hepatocitos, demostrando un uso seguro. La viscosidad de la quitosana y su nivel de hidratación fueron mayores que los del alginato, mientras que el potencial zeta de la quitosana fue altamente positivo y el del alginato negativo. Las formulaciones de Cap y las partículas de quitosana tenían tallas nanométricas, mientras que el Cap en alginato formó micropartículas que se observaron en zeta seizer y microscopios electrónicos de barrido. El perfil de liberación de las nanopartículas ocurrió de forma ascendente, extendida y controlada en comparación con el de las micropartículas. Además, el perfil de liberación de la quitosana fue superior al del alginato. Los factores esenciales a controlar en sistemas de liberación con capacidad adyuvante incluyen: partículas adyuvantes de pequeño tamaño (nano), sistemas de liberación con bajo perfil de hidratación, alta viscosidad y poder de encapsulación reversible. La quitosana ofrece una capacidad superior para la liberación del adyuvante nano-Cap. Este novedoso estudio, responde a la necesidad de optimizar las formulaciones antes de los estudios in vivo en animales, sin tener en cuenta el tamaño de partículas o la cinética de distribución.
Palabras clave: nano-adyuvante, sistemas de liberación, vacunas mucosales.
INTRODUCTION
Vaccines are preventive biological preparations; they are the only applicable and most effective pre-exposure preventive tools against most of infectious diseases in human. The immune-protective microbial epitope (s), recombinant proteins, or antigen-coding DNA is the core functional ingredient of vaccines. These antigens are the core ingredient responsible for the induction of the different post-vaccination protective immune response; cellular, humoral or mucosal immune responses.
In general, most successful vaccines protect through induction of parenteral immunity mainly (IgG) antibodies. However, protection against mucosal associated pathogens requires induction of more than one type of immune response based on the entry site and the nature infectious agent. Developing effective post-vaccination protection against either nasal or intestinal life-threatening infectious diseases; could only be achieved through inducing both systemic and mucosal immunity. The type of developed post-exposure immune response depends on the site of pathogen entry or the vaccine administration route.
Therefore, the introduction of an improved vaccine delivery system or strategies that maintains the safety issue with a capacity to improve a well-developed post-vaccination protective mucosal immunity became a priority towards mucosal associated pathogens of the digestive, respiratory or the urogenital tracts, through induction of high levels of antibodies, mainly secretory IgA at mucosal surfaces beside systemic neutralizing IgG antibodies. Examples of such pathogens include Streptococcus pneumoniae, Neisseria gonorrhoeae, Vibrio cholerae, Mycobacterium tuberculosis, Human influenza viruses, Human papilloma virus (HPV) (1).
The role of adjuvants in boosting the post-immunization response: Nowadays, vaccine delivery is one of the most strategic approaches used in modulating the post-immunization response of interest. In addition, the mucosal delivery of vaccine became on top of demands, vaccine adjuvants, or delivery carriers are the key tools to be approached for boosting measurable systemic or mucosal immunity, (2). In addition, this could be applied through incorporation of a new improved combination of vaccine with a nano size adjuvants particle encapsulated in delivery carriers. Adjuvants have already been used in vaccines to increase their immunogenicity and to induce higher levels of protective antibody compared with un-adsorbed vaccines.
Generally, adjuvants boost the overall response towards vaccines through potentiating an increased number of lymphocytes clones with minimal antigenic quantity or modulating the type of immune response(s) based on adjuvant capacity to trigger specific lymphocyte and cytokines signaling pathway(s). Adjuvants work through its binding to vaccine epitopes, peptides, or antigens, increasing their molecular weight, delaying their clearance from the circulatory system by the phagocytic cells, improving their antigenic uptake by macrophages, slowing down their clearance by the phagocytic cells, and extending their release to the immune cells. And, in turn controlling adjuvant release could prolong antigen delivery, presentation, activation of a measurable number of lymphocyte clones, and collectively will end-up with an elevated post-vaccination immune-protection. Incorporating nano-size adjuvant in vaccines not only provides an attained availability of vaccine epitope(s) but it will offer a long-lasting protection with increased number of activated lymphocyte memory cells that will expand faster when re-encountering the same pathogen and boost high levels of class specific antibody in term of affinity and avidity compared to the un-adsorbed vaccine (3, 4).
Currently, there are distinctive types of vaccine adjuvants used in human vaccine preparations with countless different properties. However, their chemical nature raises questions about vaccine safety regardless their efficacy. The current trends in vaccine adjuvants focus on developing less toxic, effective, biodegradable, and safer adjuvant or a carrier for vaccine delivery to strengthen the post-vaccination protection against most of human viral and bacterial infections (5).
The advantage of nano-size based vaccine delivery: Since its introduction, large number of Nano-biotechnology, medical applications and protocols had been developed, mainly for studying drug delivery, in which a nano-size carrier particles used to entrap the therapeutic drug and provide a sustainable, slower release of a controlled delivery, in order to achieve the similar therapeutic effect in smaller doses of a prolonged action with minimal drug side effects. Therefore, applying the same principle in vaccine delivery, combining both adjuvant and carriers of a nano-particles size in one formula; could be a useful potential first; to protect the core components of vaccines (antigen, peptides, or epitopes) against degrading effect on vaccine delivery site, such as digestive enzymes, acidity, pH or prolonged exposure to an increased temperature, (6). Secondly; the nano-adjuvants could control the release, boost and modulate the activation of lymphocyte clones to develop the required type and level of post-vaccination immune response.
Calcium phosphate used for long term as a key supplement in bone regeneration, where Cap makes up 70% of bone and 90% of neonate teeth (7). Therefore, it has been considered safe for use in other medical and biomedical applications, such as a supplement for hypokalemia, non-viral gene transfection, biological purification, and delivery of therapeutic protein products like insulin or as an adjuvant for vaccine, (8). Cap is the only nontoxic, biodegradable and non-antigenic adjuvant because it is a body ingredient, compared to other materials used in adjuvant preparation such as aluminium salts. In addition, it has been in use as an adjuvant in vaccines since 1985 (9).
Polymeric nanoparticles used for drug delivery at a lower concentration range between (0.05-1%.), there are so many different polymers used in preparing Nano-particle carriers that improve drug delivery such as gelatines, Chitosan, Alginate, Polyethylene glycol, starch and other carbohydrate derivatives (10). The use of such polymeric carriers could be one of the best choice to improve the delivery of vaccines through mucosal surfaces, where their gradually swelling, increase gel porosity that ends in a controlled release of the entrapped therapeutic protein (11, 12).
Chitosan; a poly-glucose amines, is a biodegradable and nontoxic hydrophobic polymer. Muco-adhesive and of a low solubility in water, therefore it is applied as delivery carrier (13), besides it is also used in preparation of medicinal Nano-composite and as antimicrobial wound healing films (14). Chitosan physical properties, such as viscosity, total high positive charge, particle size, adhesiveness, polymer hydrophobicity and swelling profile play a key role in its improved controlled release of drugs, therapeutic proteins like insulin or its potential application in vaccine delivery mainly towards mucosal surfaces (4, 15). The increased positive charge, which dedicated to its amino group make Chitosan attractive and superior carrier to target the negatively charged mucosal surfaces and very useful in non-viral gene (DNA) delivery (8, 15).
Alginate, a hydrophilic co-polymer, contains α-L-guluronic acid and β-D-mannuronic acid polysaccharide. It is an extract from brown Algae cell wall. It has many useful applications such as a thickening agent in food where it is able to convert a liquid into a gel form at room temperature, it also used in wound dressing, coating of the tablet, tissue engineering, and medical drug delivery (16, 17).
The importance of in-vitro monitoring model in vaccine downstream preparation: In vaccine research generally and specifically the mucosal vaccine development, up-to-date there is a gap in checking the pre-clinical in-vitro delivery (release) profile of vaccine antigens from their adsorbing adjuvants. Therefore, introducing an in vitro delivery model for monitor vaccine release profile from its adsorbing or encapsulating carrier, using in-vitro monitoring protocol is considered a crucial demand and a key tool in designing successful mucosal vaccines. In addition, it will provide an opened in-vitro platform for optimizing vaccine delivery carriers’ formulations to boost and modulate the mucosal immune response based on the interested mucosal site and vaccine administration route.
Vaccine delivery formula: One of the best formulation of choice for improving mucosal vaccine delivery could be achievable through the entrapment of adjuvants in a muco-adhesive polymeric delivery carrier as a potential and novel vaccine delivery formula. Moreover, the in-vitro monitoring of adjuvant or vaccine release from its carriers will serve as a novel and unique pre-clinical protocol for optimizing vaccine delivery profile and formulations of interest and considered as a major additive step in reducing vaccines downstream processing and developmental cost before conducting the routine direct vaccine testing animal trials without un-optimized preclinical in-vitro delivery profile.
Study aim: The aim of this study is to introduce a novel in-vitro delivery protocol for in-vitro monitoring the release and delivery profile of Nano & micro-particles of Calcium phosphate vaccine-adjuvant from their encapsulating Chitosan and Alginate polymeric carriers as a potential new delivery system for mucosal vaccines.
Study design: Calcium phosphate as safe, non-antigenic and biocompatible body ingredient, was chosen as the best material for preparing of Nano-size particles adjuvant. One adjuvant prepared in a Nano-particles size and compared to a ready-made commercial adjuvant of micro-particles size from Brenntag Biosector (Denmark). The two adjuvants used to study their in-vitro capacity in controlling the release profile from a loading carrier of Chitosan and Alginate in a designed in vitro adjuvant delivery model as follows: in-vitro delivery model mimicking oral mucosal permeability, temperature and pH environment were developed using an artificial semi-permeable membrane, the dose response over time used to study both swelling of the delivery carriers and the adjuvants-release profile from their loading two carrier Hydrogels.
MATERIALS & METHODS
Materials: Most of the materials used such as; Calcium phosphate, Chitosan of medium molecular weight (75-85% deacetylated, Cas. Number 9012-76-4), sodium Alginate, (Cas Number 9005-38-3), phosphate buffer tables, dialysis tubes; were bought from Sigma, semipermeable membrane of a mean pore size range between 90-110 nm based on the SEM result from (Spectrum Labs, Taiwan) and Calcium quantification kit OCPC kit from (Reckon Diagnostics Pvt. India).
Preparation and characterization of CAP adjuvant: Preparation: Calcium phosphate (Sigma, USA) 10 mL volume prepared as follows 10 mg/mL (w/v) of the powder dissolved in deionized water; the adjuvant was mixed in a vortex for 3-4 minutes; stirred at room temperature for 90 minutes and sonicated for 45 minutes. Another ready for use commercial CaP adjuvant; bought from Brenntag Biosector, (Denmark), and examined for particle morphology, size, and Zeta-potential.
Preparation of polymeric hydrogels: Chitosan was prepared in gel form as follows; 3% concentration of Chitosan solution (3 mg/mL (w/v)) dissolved in 20 mL volume of 1% of acetic acid in deionized water (v/v), mixed on vortex for 3-4 minutes, centrifuged at 2000 rpm at 4°C for 5 minutes to remove the air. Aliquots of 40 mL were sterilized by autoclave at 121°C for 15 minutes, then the dense gel stirred for three hours and sonicated at high voltage for 15 minutes. The sterile gel preserved at 4°C until used for characterizing tests.
The Alginate prepared in a gel form as follows: 3% concentration of a 20 mL Alginate solution (3 mg/ mL (w/v)) dissolved in 37°C warm deionized water mixed in a vortex for 10 minutes, centrifuged at 4000 rpm at 4°C for 5 minutes. Aliquots of 40 mL tubes, autoclaved at 121°C for 15 minutes, then the dense gel was stirred for 3 hours and sonicated at high voltage for 15 minutes. The sterile gel was stored at 4°C until used for testing for particle characterization.
Physic-chemical properties: Samples of the prepared adjuvant tested for viscosity in digital refract meter (Model AR2008, Kruss, Germany), pH, particle morphology SEM, TEM, particle size, and Zeta-potential measured in Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK).The adjuvant and polymeric hydrogels were examined for Carbon Hydrogen Nitrogen Sulphur content; C.H.N.S (LECO, model CHNS-932, USA) instrument. Samples were also examined in Atomic Absorption Spectroscopy; A.A.S for Ca & Na atoms.
Polymers and adjuvants cell cytotoxicity: The cytotoxic effect of the adjuvants was examined in human liver cells after incubation with different concentrations of each of the two adjuvants starting with 1.5 mg/mL; each adjuvant dilution was done in four replicates. The samples were diluted in free serum RPMI-1640 cell culture medium and incubated with Hep-G2 cell line monolayers in 96 well microplate. The plates were incubated at 37°C, in 5% CO2, for 48 hours with untreated cell as a relative control of (100%) cell viability. The plates were treated with 15 µl of 5 mg/mL Tetrazole solution (Sigma, Cas. Number 298-93-1, USA) including the control cell, the plates were incubated for 3.5 hours at 37°C in in 5% CO2. The culture media were removed, and the plates were carefully washed with sterile PBS and 150 µL of DMSO were added to dissolve the enzymatic-Tetrazole reaction precipitate, incubated for 15-18 minutes, the plates were agitated and the absorbance read at 590 nm main filter with 620 nm as reference filter. The mean cell viability was calculated in percentage according to the following formula: Cell viability = [Mean O.D of the experimental sample/mean O.D of the control group (after subtracting the reading of blank wells) × 100%].
Adjuvants and carrier gels; pH, Viscosity & Density: pH was measured in a digital hand pH-meter (Sigma (USA) to the Chitosan and Alginate. Samples of Chitosan and Alginate prepared (0.5, 1 and 2.5%) were examined for viscosity in a rheometer (Rotovisco, Germany). The density of CAP, Chitosan & Alginate different preparations (0.5, 1 and 2.5 %) was measured by a refract meter and recorded (r.i). The obtained results were plotted against each sample concentration in Minitab-16 software.
Size and Zeta-potential of the adjuvant and hydrogels: Three aliquots of 3 mL samples of the prepared and commercial Calcium phosphate adjuvants (3 mg/mL), Chitosan (3%), Alginate gels (3%) were used to examine the particle size distribution and zeta potential using Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK).
Transmission Electron Microscopy (TEM): Sample volume of five to seven microliters of the preparation and commercial Calcium phosphate, Chitosan and Alginate gels were added to sample holder, and allowed to dry for 25-30 minutes, covered with a negative stain and examined under transmission electron microscope for the particle morphology and size (LEO 912 AB Energy Filtered Transmission. Electron Microscopy (EFTEM) (Carl Zeiss Inc. Germany).
Field Emission Scanning Electron Microscope (FESEM): Small volumes of five microliters from each the two adjuvants were added to the sample holders, after drying for 30 minutes, then samples were gold coated under a vacuum and micrographs were taken under (FESEM; FEI-NOVA NanoSEM 230, Japan) microscope. While the Chitosan and Alginate samples of 1 mL were freeze-dried and the dry sample were added directly to the sample holders, and examined directly without staining for their particulate’s morphology and size under a Scanning Electron Microscope (JEOL JSM6490A, Japan) at a voltage of 20 V.
Preparation of polymeric hydrogels loaded adjuvant: The prepared and commercial Calcium phosphate were entrapped into the Chitosan and Alginate hydrogels as follows: 0.3 mL Nano-Calcium phosphate (10%) added to 2.7 mL of each Chitosan and Alginate gels (3%) at a final concentration of adjuvant 1 mg/ mL of each gel. 1 mL of the commercial Calcium phosphate (3%) was added to 2 mL of each Chitosan and Alginate gels (3%) at a same final adjuvant concentration of 1 mg/mL.
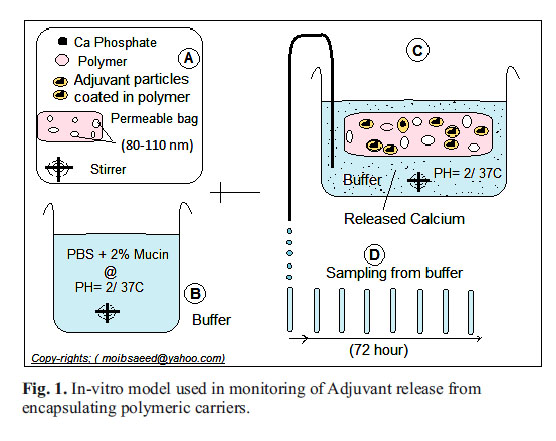
In-vitro carriers swelling & adjuvants monitoring model: The core part of this model is the semi-permeable dialysis tube (spectrum labs, Taiwan), that mimics the permeability of the lining of mucosal surface layers of epithelial cells. It is a weak acid-mucin buffer as follow: (Mucin powder (Cas number 84082-64-4, Sigma-Aldrich) dissolved at concentration of 2%, in phosphate buffered saline (PBS) of a pH 2-3 adjusted with hydrochloric acid under conscious stirring for 60 minute at room temperature. In this development model, 1 mL from each the prepared mixture of adjuvant and carrier were added to a dialysis tube, and each bag was inserted into a well in six-well plate. The well filled with normal saline as isotonic buffer of weak acidic pH 2 was adjusted with HCl, incubated at 37°C water bath under continuous slow agitation at 10 rpm (Fig. 1).
Monitoring hydrogel swelling profile loaded Adjuvant: Nano and micro particles of Calcium adjuvant were loaded into Chitosan and Alginate gel as follows: 3 mL (3%, 2 %, & 1%) of each of both types of gel -gels in a 15 mL tube, at room temperature with continuous shaking. While the gel was running on a vortex, 0.3 mL of nano and 1 mL of micro-size adjuvant were successively added in drops to the gel preparations. Besides, two preparations of gel-free adjuvant, swelling profile monitored by adding 1 mL from each the prepared mixture of adjuvant and carrier added to a dialysis tube (12-14 cm, Sigma), then the tubes sides were sealed without leakage and each tube was inserted into a well of six-well plate. The well filled with normal saline as isotonic buffer of acidic pH 1-2 incubated at 37°C water bath under continuous slow agitation at 10 rpm. At a time interval, each tube was removed, dried from outside and weighed with a digital electronic sensitive balance. The time interval was at 1/2, 1, 2, 3, 6, 18, 24, 28, 72, & 96 hours. The increase in sample weight was recorded, divided by the original pre-weight record, and calculated as increase of weight in percentage as the best indication of gel swelling profile.
Study of adjuvant release from the encapsulating carrier hydrogels: Similar combinations of adjuvants entrapped in the carrier gels were prepared and examined for the release of Calcium particles to the outer buffer, across the pores of enclosing semi-permeable membrane, over the experiment time of 96 hours as follows: The adjuvant-loaded on the carrier gel of 1.5 mL volume was enclosed in a semipermeable dialysis membrane inserted in a 15 mL tube, containing phosphate buffer of pH 2, at 37°C, and under continuous slow agitation at 15 rpm. Calcium quantification kit was used to measure the released adjuvant over the time interval from each preparation. Unloaded Nano and micro particles of Calcium phosphate were used as adjuvant controls.
Electron-micrographs of in-vitro release permeable membrane: Samples of the dialysis membrane pre and post-release of nano and micro adjuvant were examined with SEM to view the effect of the release process on membrane pore size.
Statistical analysis: Samples’ triplicates were statistically analysed in student t-test and one-way ANOVA. Statistical tests were used to analyse swelling and adjuvants release data of the different formulations in Minitab over 16.0 (Minitab Inc, USA), and the p-value <0.05 was considered significant.
RESULTS
Properties of the nano and micro-particles adjuvant: Calcium phosphate revealed high content of Calcium ion, lower nitrogen and carbon when examined for C.H.N.S assay (Fig. 2).
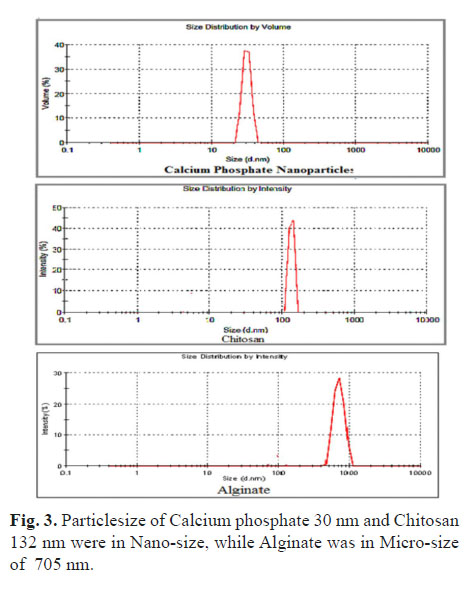
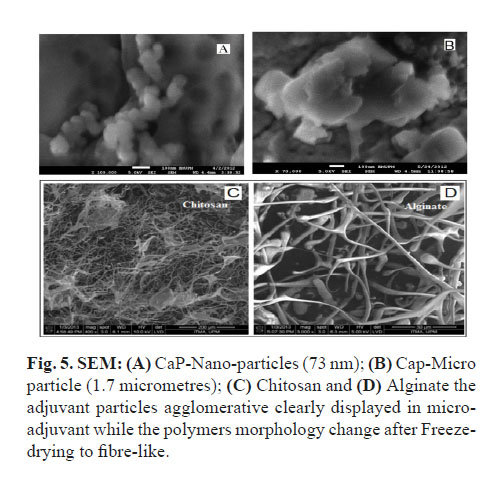
Calcium phosphate was in nano-size of 75 nm under SEM and 30 nm in Zeta-Sizer with negative Zeta-potential of (11.2 -mv); while the commercial adjuvant showed more negative Zeta-potential and bigger particle size up to 1.7 micrometre (Fig. 3, 4, 5 and 6). The viscosity of the nanoparticle Calcium phosphate was similar to that of the water in different adjuvant concentrations ranging from (0.5 - 2.5%) (Fig. 7-A).
Hydrogels Viscosity: Increased refractive index (density) of the hydrogel was proportional to the increased viscosity and Chitosan was more viscosity than Alginate as appeared at the 2.5% gel concentration (Fig. 7-A, B, and C).
Properties of adjuvants and delivery carriers’ particles: The gel made of Chitosan polymers was prepared in 1% acetic acid showed higher content of nitrogen because of the presence of amino group in Chitosan that is responsible for water insolubility of the polymer. While the other polymeric gel of Alginate, contains low Calcium and nitrogen with high content of sodium ion that increases its water solubility.
The polymer showed a difference in particle size and major variation in their Zeta-potential, the Chitosan was in nano particle size of 132 nm with a high positive charge up to +mv 71.8, but Alginate gel was at a micro-particle in size (705 nm) with a negative zeta potential up to -mv 43. Under SEM, the liquid samples of both gels formed a heat sensitive film that was difficult to visualize while after freeze-drying, the polymers appeared as in fibres or filament morphology (Fig. 5-C and 5-D). The viscosity of the polymer was as expected, higher than that of water due to their increased density, but Chitosan was more viscous compared to Alginate hydrogel throughout the different preparations ranging from 0.5 to 2.5%, (Fig. 7-A). The pH of Chitosan was a bit lower, (pH 4.8) than that of Alginate (pH 7.6) due to the use of weak acetic acid as solvent for Chitosan.
The zeta potential of Calcium loaded in Chitosan was in less positive charge compared to that, the high positivity of the un-loaded Chitosan (+78.2 mv) that remained positive post-encapsulation of the negative adjuvant with the mixture Zeta-potential of (+32.7 mv) (unpublished data). Nevertheless, the reverse took place when CaP encapsulated in Alginate where they remain negative Zeta-potential and the size increased compared to that of the single Calcium adjuvant or the Alginate polymer (Fig. 4).
Cytotoxicity: The cytotoxicity of adjuvant and the polymers on human liver HEP-G2 cell line in cell viability were assayed (enzymatic method). Alginate appears less toxic than Chitosan preparation, which anticipated to the adverse effect of Chitosan’s solvent acetic acid. The micro adjuvant showed less toxicity than the nano adjuvant because of the differences in size where the nano size may precipitate and reduce the cell membrane permeability, but more than 50% of the cells were still alive in a nano treated cell and were considered nontoxic (Fig. 8).
Swelling profile of the gel-loaded adjuvants: The polymeric hydrogels examined for their swelling properties of higher gel density (3%) in both un-loaded form and in a mixture with the Nano and micro-size adjuvants.
In-vitro swelling of Chitosan was lower than Alginate at the early 3-4 hours (Fig. 7-B). But, when swelling was examined for a prolonged incubation time, Chitosan showed an elevated extending swelling exceeding Alginate at longer incubation time of 72 hours (Fig. 9) as a direct impact of its difficult solubility, and slow water absorption anticipated to the presence of the amino group in Chitosan compared to Alginate’s faster solubility enhanced its water absorption leading to a fast saturation and end point of the polymer’s particles swelling. While, Chitosan’s hydrophobicity slows its particles saturation, and the process continues in ascending manner (Fig. 9).
Among the adjuvant loaded in carrier polymers swelling profile displayed by Alginate loaded micro-particles of CaP adjuvant were minimal than its similar particles loaded in Chitosan. Moreover, the Nano-size Calcium adjuvants loaded in both carriers and micro-particles loaded in Chitosan was elevated. Chitosan loaded Nano-adjuvant displayed little increased swelling compared to the similar adjuvant loaded in Alginate. The Micro-adjuvant loaded Chitosan hydrogel showed elevated swelling compared to that its similar particle size loaded in Alginate. It could be attributed to effects of adjuvants particle size and Zeta-potential to the reversibility and strength of Calcium-Alginate crosslinking (Fig. 3, 5, and 9).
The in vitro release profile of calcium phosphate adjuvant from the encapsulating carrier-gels: The adjuvants loaded into the carrier gels incubated in a buffer physically closer to the mucosal pH 2; temperature 37°C, in the physiological phosphate buffer and the mixture kept under continuous slow agitation. Samples were drown at intervals and the Calcium contents were assayed in (OCPC) Calcium quantification reagent (India). The adjuvant release profile showed high release among the Calcium loaded in Chitosan hydrogel compared to that of the Alginate, the Nano Calcium adjuvant was the highest release during the early 18 hours. Nano in Alginate was the slowest release, with almost similar release between the micro-size adjuvant from both hydrogels (Fig. 10).
Chitosan continuously improves the release of a nano-adjuvant >70%, and 60% of a micro-adjuvant at 24 hrs.; whilst Alginate irreversible interaction with the loaded Calcium and reduces its release of Nano-adjuvant below (45%), the micro particle adjuvant release from Alginate (25-30%) and the release of unloaded adjuvant around 15% due to its larger particle size (Fig. 10).
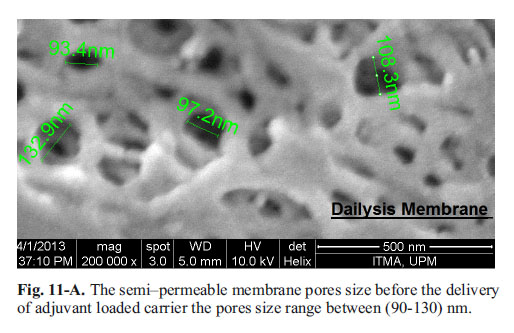
The release of the adjuvant from the gel across the semipermeable membrane pores are shown in the micrograph; (Fig. 11-A, B and C). It seems that the Nano-size Calcium can easily pass through the membrane pores that were shown in its high release from the Chitosan physical mixture. While it is a chemical interaction with Alginate does not allow the nanoparticles to swell out of the gel, been released and cross the membrane the particles passed and the membrane pores remain open. However, apart from Calcium and Alginate chemical bonding, their larger particle size does not allow the adjuvant movement across the permeable membrane; otherwise, its particle adhered and blocked the membrane pores.
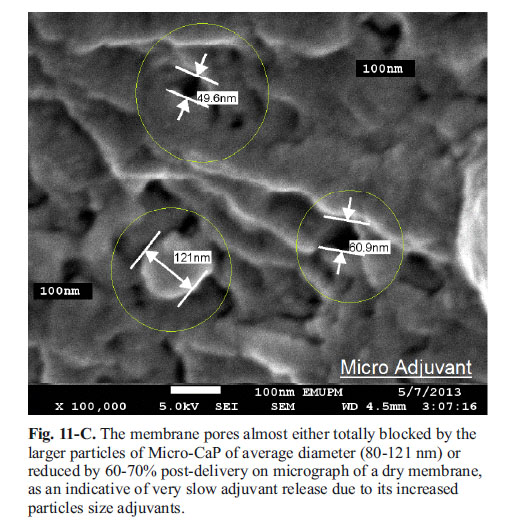
Adjuvant particles in-vitro release mechanics (SEM Micrograph): Post-release SEM image of semi-permeable membrane: The micrograph shows the pore of the dialysis semi-permeable membrane before its use in release ranges between (90-110 nm). The larger micro particle adjuvant was trapped in the membrane pores and its pores either reduced 80% or completely blocked, therefore the micro-adjuvant release was very slow and only 10-15% delivered. Whilst, the nano-particles adjuvant easily crosses through the membrane, and this results in an improved delivery 40% in Alginate and ascending sustained up to 70% in Chitosan.
DISCUSSION
Alginate appears less toxic than Chitosan preparation, which anticipated to the adverse effect of Chitosan’s solvent acetic acid. The adjuvant Zeta-potential remained negative in spite of the major difference in the particle size between the two adjuvants, and the negative Zeta-potential is mainly attributed to its phosphate group.
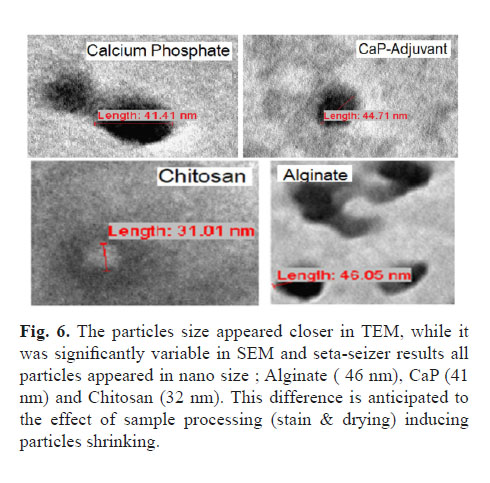
The use of Zeta-Sizer and SEM, for particle size measurement showed a real variation in particle size. In liquid samples, adjuvants were different in size compared to that in TEM due to variability in the form of samples used. But, both electron micrographs remain the best option to study adjuvant particle morphology (Fig. 5-A and 5-B).
The polymer swelling, revealed an inversely proportionate correlation to their concentration; it was significantly slower at an increased polymeric gel concentration depending on they are water miscible and the degree of hydrophilicity (Alginate) and hydrophobicity (Chitosan). It seems that the high viscosity of Chitosan compared to Alginate has a direct impact on its swelling profile, in addition; the irreversible crosslinking of Calcium-alginate halted the polymer swelling, mainly it appeared in CaP micro-particles which resulted in at least swelling compared to Alginate loaded with Nano-particles. The incorporation of adjuvant in polymer carriers leads to delay on swelling (Fig. 9).
During the swelling process the polymer particles absorb water and release the loaded particles until polymer saturation occurs ending the particles release, therefore, based on the solvent, viscosity and hydrophobicity of Chitosan, its particles swelling at a delayed and extended manner were in agree with the steady ascending and attained release of nano-adjuvants. In accordance to that, Chitosan also showed a controlled release of micro adjuvant. The polymer concentration, hydrophobicity and delayed swelling are Chitosan’s key properties that led to its delayed release of loaded adjuvant particles and it appeared steadily increasing in nano-adjuvant compared to micro-particles, (18). Moreover; this could help in extending the release of adsorbed vaccine and positively boosting the immune response.
Micro-particles CaP adjuvant does not show any advantage over its nano-size adjuvant. Its release was in a very slow manner, which could lead to their excretion without achieving the required trans-mucosal uptake, due to the larger particle size as appeared in SEM micrograph inhibiting its delivery across the permeable membrane pores to the outer surrounding buffer (Fig. 11-A, B and C).
Nano-size CaP adjuvant loaded carriers displayed better swelling and in-vitro release profile from both encapsulating hydrogels compared to micro particles. While the chemical irreversible interaction of Calcium with Alginate markedly reduced the release of Nano-adjuvant compared to Chitosan. The release and swelling of micro-particles adjuvant loaded in Chitosan was much better than Alginate. It seems that larger adjuvant micro-particle size increases the degree of Alginate crosslinking stabilizing the adjuvant inside the stiffly gel, resulting in a declined Alginate swelling degree and significantly inhibited adjuvant release. The importance of using Chitosan for the delivery of CaP adjuvant is indicated by its capacity to deliver nanoparticles adjuvant in a prolonged and stable manner. The negative charge of both Calcium and Alginate that could adversely affect vaccine epitopes uptake by M-cell on the other negatively charged sialic and mucin on the mucosal epithelial linings, which could lead to repulsion phenomena resulting in a reduced vaccine trans-mucosal delivery (19).
The valuable properties of Chitosan such as its physical reversible encapsulation, slow swelling and highly viscous similar to what was observed by Lehr CM, et al in 1992, (20); made this polymer very convenient and superior delivery carrier of choice at mucosal surfaces of a negative charge due to mucin contents. Therefore, Chitosan showed better physical functionality than Alginate in terms of viscosity, swelling, particles-size, Zeta-potential and as a delivery carrier revealed a homogeneous reverse interaction with the CaP adjuvant. At increased concentration, Chitosan’s delivery of nano-adjuvant improved in a stable, sustained ascending manner over an extended time, as a promising carrier for use in mucosal vaccine delivery where the dose intervals of vaccines are much longer (weeks or months) apart compared to hours intervals used in therapeutic drugs. In general, the hydrophobibicty in Chitosan’s made Chitosan one best choice of delaying the release and extending mucosal vaccine availability when delivered through the oral route, Chitosan in the range between 1-3 % could be used for urogenital vaccines, and for the delivery through nasal mucosa, a lower polymer concentration of 0.5-1% could be suitable.
Alginate appeared as an unsuitable carrier for ionic adjuvants delivery, such as (Ca++) compared to Chitosan, especially at increased concentration of either Alginate or Calcium or increased adjuvant’s particle-size. Alginate was only capable to deliver nano-Calcium adjuvant at lower concentration due to their minimal crosslinking irreversible interaction, which could take place with other cationic adjuvants or vaccine preparation containing cations like Calcium or magnesium, the common buffering solution ingredients. Therefore, the use of Alginate for calcium adjuvant delivery seems not suitable and needs extensive effort to optimize and improve its capacity of releasing the adjuvant, due to the formation of irreversible displacement of sodium with Calcium in Alginate resulting in Calcium-Alginate chemical Crosslink, which led to a significant drop in polymer swelling and adjuvant release compared with Chitosan (21).
CaP has been successfully used as adjuvant in many bacterial (Diphtheria, tetanus & BCG) and viral (yellow fever, measles, polio & Hepatitis-B) human vaccine vaccines. CaP biocompatibility remained the major advantage. Its weak toxic effect examined on the in-vitro proliferation of human liver cell (Fig. 8), and in other studies, and its minimal induction of IgE antibody class, indicate that it is well tolerated by the body immune system An efficient vaccine adsorption is achieved, and slow antigen release compared to alum adjuvant, as reported by Relyveld, E.H. (1986) (9).
Nano-size Calcium phosphate adjuvant could be a useful alternative as a very safe, biocompatible, non-antigenic adjuvant for systemic vaccine administration compared to its micro-particles size or currently used adjuvants, due its increased total adsorbing surfaces, an area offering a prolonged vaccine epitopes release that will help in extending lymphocyte stimulation. In addition, the encapsulation of the nano Calcium phosphate adjuvant-adsorbed vaccine in a positively charged, hydrophobic, muco-adhesive, and biodegradable Chitosan carrier gel; offers an ideal mucosal carrier to improve vaccine epitopes uptake, transmucosal delivery by M-cell and enhancing antigenic presentation through phagocytes and dendritic cells at subcutaneous. Therefore, Chitosan could be useful potential for the delivery of the Calcium phosphate, other adjuvants and vaccines through mucosal surfaces of the respiratory, digestive, or urogenital tract vaccines. And this formulation could also be applied in therapeutic drug delivery such as osteoporosis, tissue engineering and treating hypokalemia.
CONCLUSIONS
As a result, the major key factors that directly affect and delay the release profile of the vaccine-adjuvant delivery includes; slow rate of swelling, its high viscosity, the use of a nano-size adjuvant and carrier, in addition, to physical reversible entrapment nature. Introductions of in-vitro delivery protocol firstly; it will open doors for monitoring adjuvants and vaccines release kinetics; secondly, it will help in optimizing mucosal vaccine formula of interest. In addition, it will reduce the cost and time of optimizing the quantity of adjuvant, carrier, or vaccine in the final vaccine preparation before advancing to the in-vivo delivering vaccine in animal trial without knowing the best required vaccine formula of choice.
These novelties of developing in-vitro adjuvant release, vaccines monitoring protocol and combining of a safe, non-antigenic, nano-size particulate adjuvant in a muco-adhesive nano-size Chitosan carrier for delivering mucosal vaccine as a novel carrier formulation to be approached, could offer major benefits in boosting mucosal immune response towards vaccines. The first preclinical in-vitro protocol was developed for optimizing mucosal vaccine design, at the preparative downstream processes delivery before its usual direct testing in animal trial regardless to its adjuvant particles size or delivery kinetics.
In addition, such in-vitro vaccine monitoring will open the doors for formulating effective, applicable and affordable mucosal vaccine delivery system of choice targeting the interested mucosal surfaces on the nasal, oral or urogenital routes compared to the current formulated mucosal vaccines.
REFERENCES
1. Baudner BC, Morandi M, Giuliani MM, Verhoef JC, Junginger HE, Costantino P, et al. Modulation of Immune Response to Group C Meningococcal Conjugate Vaccine Given Intranasally to Mice Together with the LTK63 Mucosal Adjuvant and the Trimethyl Chitosan Delivery System. The Journal of Infectious Diseases 2004;189:828-32.
2. Illum L, Jabbal-Gill I, Hinchcliffe M. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Del Rev 2001;51:81-96.
3. Gupta RK, Relyveldt RH, Lindbladt EB, Bizzini B, Ben-Efraim S, Gupta CK. Adjuvants: a balance between toxicity and adjuvanticity. Vaccine 1993;11(3):293-306
4. Cox JC, Coulter AR. Adjuvants: a classification and review of their modes of actionVaccine 1997;15(3):248-56.
5. Galindo-Rodríguez SA, Allemann E, Fessi H, Doelker E. Polymeric nanoparticles for oral delivery of drugs and vaccines: a critical evaluation of in vivo studies. Crit Rev Ther Drug Carrier Sys 2005;22:419-64.
6. Calvo P, Remunan-López C, Vila-Jato JL, Alonso MJ. Novel hydrophilic Chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 1997;63:125-32.
7. Lucey JA, Horne DS. Milk Salts: Technological Significance. In: McSweeney PLH, Fox PF, editors. Advanced Dairy Chemistry 3-Lactose, Water Salts, and Minor Constituents; 3rd ed. New York: Springer; 2009.p.351-90.
8. Chernousova S, Klesing J, Soklakova J, Epple M. A genetically active nano-Calcium phosphate paste for bone substitution, encoding the formation of BMP-7 and VEGF-A. RSC Adv 2013;3:11155-61.
9. Relyveld EH. Preparation and use of Calcium phosphate adsorbed vaccines. Dev Biol Stand 1986; 65:131–6.
10. Dimitrov DS. Therapeutic Proteins. In: Voynov V, Caravella JA, editors. Methods and Protocols, Methods in Molecular Biology. New York: Springer; 2013.p.1-26.
11. Ladet S, Laurent D, Domard A. Multi-membrane hydrogels. Nature 2008;452(7183):76-9.
12. Janes KA, Calvo P, Alonso MJ. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Del Rev 2001;47(1):83-97.
13. Boddupalli BM, Mohammed ZNK, Nath RA, Banji D. Mucoadhesive drug delivery system: An overview. J Adv Pharm Technol Res 2010;1(4):381-7.
14. Hu L, Sun Y, Wu Y. Advances in Chitosan-based drug delivery vehicles. Nanoscale 2013;5(8)3103-11.
15. Scherließ R. In vivo evaluation of Chitosan as an adjuvant in subcutaneous vaccine formulations. Vaccine 2013;31(42):4812-9.
16. Longer MA, Cheng HS, Robinson JR. Bioadhesive polymers as platforms for oral controlled drug delivery III: oral delivery of chlorothiazide using a bioadhesive polymer. J Pharm Sci; 1985;74(4):406-11.
17. Rawat, M. Singh, D. Saraf, S. et al. Development and in vitro evaluation of Alginate gel-encapsulated, Chitosan -coated ceramic nanocores for oral delivery enzyme. Drug Dev. Ind. Pharm; 2008;34:181-8.
18. Xing J, Deng L, Dong A. Chitosan/Alginate Nanoparticles Stabilized by Poloxamer for the Controlled Release of 5-Fluorouracil. Journal of Applied Polymer Science; 2010;117(4):2354–9.
19. Di Martino A, Sottinger M, Risbud MV. Chitosan: A versatile biopolymer. Biomaterials; 2005;26:5983–90.
20. Lehr CM, Bouwstra JA, Junginger HE. In vitro evaluation of mucoadhesive properties of Chitosan and some other natural polymers. Int J Pharm 1992;78:43-8.
21. Alves-Cardoso D, van den Beucken JJJP, Both LLH, Bender J, Jansen JA, Leeuwenburgh SC. Gelation and biocompatibility of injectable Alginate–Calcium phosphate gels for bone regeneration. J Biomed Mater Res Part A 2014;102(3):808-17.
Recibido: Noviembre de 2014 Aceptado: Febrero de 2015