Introduction
Rabbit hemorrhagic disease (RHD) is a highly virulent viral disease of Oryctolagus cuniculus which threatens the rabbit population in Egypt and worldwide. The etiological agent is the rabbit hemorrhagic disease virus (RHDV), a single-stranded positive-sense Ribonucleic acid (RNA) virus belonging to the genus Lagovirus, family Caliciviridae.1) The virus was firstly diagnosed by hemagglutination (HA) test using human type "O" red blood cells (RBC), but this test is not reliable for virus diagnosis, because non-hemagglutinating isolates of the virus.2 The virus cannot be cultivated in cell cultures, therefore virus detection and characterization were carried out by inoculation of susceptible rabbits and reverse transcriptase polymerase chain reaction (RT-PCR).3) The P2-subdomain of VP60 protein (regions C to E) is the immunodominant region of the calicivirus and changes in this region are responsible for antigenic variation.4 Based on the phylogenetic analysis of VP60 gene of pathogenic RHDV strains, they have been divided into three groups: the “classic RHDV” with the genogroups G1-G5 isolated from 1984 onward, the antigenic variant RHDVa/G6 identified in 1996, and RHDV2 identified in 2010.5 In Egypt, the virus was introduced in 1991 in Sharkia province, and then spreaded in several governorates causing high economic losses.6 The RHDVa variant strains were detected at 2006 and replaced the classic RHDV strain in vaccine manufacture starting from 2008.7 A newly emerging RHDV called RHDV2 caused sporadic outbreaks in vaccinated flocks.8 The RHDV2 infection have a variable mortality rate ranged from 5-70% with an average mortality of 20% in experimentally infected rabbits; death can occur in adult and lactating rabbits from 15 days of age.9 However, recent researches have demonstrated significant increase in RHDV2 pathogenicity within a few years.10 The current policy of RHD infection control, depends mainly on vaccination of rabbits with approved commercially available RHD vaccines in Egypt, for example, bivalent Servac RHDV and Curnipravac vaccine.7 Despite the availability of RHDV vaccines, many outbreaks have been recorded during last years that have been attributed to there’s no cross-protection immunity between RHDVa and RHDV2. Subsequently, World Organization for Animal Health (OIE) in 2018,5 advised vaccinating rabbits with vaccines that contain both antigenic types (RHDVa and RHDV2) or that contain the strain homologous to the one identified during the outbreak. This study was conducted for isolation and full characterization of newly emerging RHDV2 from suspected cases and to evaluate protective efficacy of prepared inactivated vaccine containing RHDV2 adjuvanted with Montanide ISA 206 or aluminum hydroxide gel.
Materials and Methods
Experimental rabbits
Two hundred and forty-three seronegative New Zealand White rabbits, males, weighing 1.5-2.5 kg, were purchased from a conventional rabbit try in Qalubia governorate as 45 days of age. They were reared and housed in stainless steel cages with free access to water and food and kept under observation during a 24 h acclimatization period. Rabbits were required for virus isolation, determination of lethal dose50 (LD50) and vaccine evaluation.
Reference virus
It was used a local RHDV2 strain isolated and characterized from infected flocks in Egypt and submitted to gene bank as (RHDV\Vet-Abotaleb) under accession number MN276176.8) It was kindly supplied by Reference Strain Bank Department in Central Laboratory of Veterinary Biologics and used as a positive control for virus detection by conventional RT-PCR assay.
Sample collection and preparation
A total of 11 liver samples (polled samples from each infected farm) were collected from freshly dead rabbits raised in El-Qalubia governorate in 2019. It was done according to OIE, 2018.5 The tested viral samples were named from R1 to R11.
Isolation and characterization of emerging RHDV2
Molecular identification of RHDV by conventional one-step RT-PCR assay
Viral RNA was extracted using QIA quick extraction kit (Qiagen Inc. Valencia CA) according to the manufacturer’s instructions. RT-PCR technique was performed to amplify the E-F HVRs of VP60 gene using the Superscript one step RT-PCR kit (UK), the forward primer (P33): 5’CCA CCA CCA ACA CTTCAG GT’3 and the reverse primer (P34): 5’CAG GTT GAA CAC GAG TGT GC ’3. It was followed the thermal profile: RT step at 50ºC for 30 min, initial denaturation at 95ºC for 15 min, then 40 cycles of 95ºC for 1 min, 56ºC for 1 min, 72ºC for 2 min, and a final extension step at 72ºC for 10 min, on thermocycler (Biometra, Germany) according to Fahmy et al.11 The PCR products were loaded into 1.5% agarose gel (molecular biology grade) electrophoresis and visualized by ultraviolet transillumination with ethidium bromide stain (0.5 μg/ mL) to show amplification at 538 bp.
Virus inoculation in susceptible rabbit
Ten liver samples positive to RHD by PCR were inoculated in 20 seronegative susceptible rabbits (one sample/two rabbits) at the age of 3 months, weighting 1.5 to 2 kg. Two rabbits were kept as uninfected controls. The mortality (%) was recorded with the HA activity for each isolate.12
Sequencing and phylogenetic analysis
Based on HA titer and RT-PCR results, two positive samples were selected for sequencing and genetic characterization. The amplified DNA fragments of the two selected isolated samples were purified using QIA quick gel extraction kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. It was followed by sequencing, using Big Dye Terminator V3.1 cycle sequencing kit (Perkin-Elmer, Foster city, CA) according to the manufacturer’s instructions, the VP60 specific primers11 for RT-PCR and the Applied Biosystems ABI 3500 genetic analyzer (Life Technologies, California, USA). The samples were published in the gene bank (A/Qalubia/2019 and B/Qalubia/2019) under the accession number (MT07629 and MT067630). Nucleotide and amino acid sequences of the two isolates were aligned with sequences of RHDV strains, corresponding to different groups (G1-G6, RHDV2), obtained from the National Center for Biotechnology Information. The alignment was carried out using the CLUSTAL-W program and the Meg Align module of DNASTAR software (Laser gene version 7.2; DNASTAR, Madison, WI, USA). The phylogenetic tree was constructed using MEGA version 7 (www.megasoftware.net) by maximum likelihood tree method with moderate strength and 1000 bootstrap replicates. The pairwise nucleotide and amino acid identity percent was calculated using DNA star software (DNA Star, Madison, WI).
Determination of LD50
Fifty susceptible rabbits were used for calculation of LD50. This test was carried out according to OIE, 2018.5
Preparation of the candidate vaccine
The Virus
Local Egyptian strain of RHDV2 designated as (A/RHDV2/Qalubia/Egypt/2019) with accession number MT07629, titer of 108.2 LD50/mL and of HA titer equal to 213 HA unit was used for vaccine preparation, challenge test and homologous antigen with a final concentration of 8 HA/mL in Hemagglutination inhibition (HI) test.
Candidate vaccine preparation
The RHDV2 vaccine was prepared according to OIE, 2018.5 Briefly, the supernatant from one RHDV2 isolate, was taken and inactivated using formalin with a final concentration of 2% of the total volume for 48 h. Assessment on virus inactivation was achieved through injection of five rabbits with inactivated suspension and keeping two rabbits as controls. If the inoculated rabbits did not show any clinical signs of the disease or mortality subsequently; the inactivated suspension was considered ready for emulsifying with the vaccine adjuvant. The suspension was adjuvanted with aluminum hydroxide gel 2% (to occupy 25% of vaccine volume) or Montanide ISA 206 oil (to occupy 50% of preparation volume). The recommended vaccine dose (0.5 mL/animal) contained 210 HAU per vaccine dose, was inoculated subcutaneously (S/C).5,13
Evaluation of two prepared vaccines containing rabbit hyemorrhagic disease virus 2 adjuvanted with aluminum hydroxide gel or Montanide ISA
A total of 162 susceptible rabbits were used for sterility, safety and potency tests which were done according to OIE, 2018.5
One hundred and fifty rabbits were equally divided into three groups. The first group (1st group) was vaccinated S/C with 0.5 mL inactivated aluminum hydroxide gel adjuvanted vaccine; the second group (2nd group) was vaccinated S/C with the inactivated oil adjuvanted vaccine with the same dose and the third group (3rd group) was control unvaccinated group. Individual blood samples were collected from 10 rabbits of each group weekly, between the first and fourth weeks after vaccination and RHDV2 HI antibodies were measured in each collected serum sample by HI test. Ten rabbits from each group were challenged intramuscularly at a dose of 1 mL/rabbit by 100 LD50 of local RHDV2 after each week post vaccination. Observations for clinical signs and death were carried out for 14 days post challenge. Serum samples from survived rabbits were collected at 1 and 2 weeks post challenge for the HI test.
Protection (%) = Number of survivals/Total number of challenged rabbits X 100
Results
Slide and micro-plate HA test
The microtiter plate HA test revealed that 10 out of 11 tested viral samples were HA positive, with titer values ranged from 8-13 log2 as shown in Table 1.
Table 1 HA titers of prepared viral samples before and after passage in susceptible rabbits.
| Sample code number | Slid HA result | Viral HA titer by microtiter HAT (log2) | |
|---|---|---|---|
| Original (HAU) | First passage (HAU) | ||
| R1 | positive | 9 | 11 |
| R2 | positive | 10 | 12 |
| R3 | negative | 0 | 0 |
| R4 | positive | 11 | 11 |
| R5 | positive | 12 | 13 |
| R6 | positive | 13 | 13 |
| R7 | positive | 12 | 11 |
| R8 | positive | 13 | 13 |
| R9 | positive | 9 | 9 |
| R10 | positive | 8 | 9 |
| R11 | positive | 11 | 12 |
| R: Symbol for isolation code number. HAU: Hemagglutination unit. HAT: Hemagglutination test. | |||
Molecular identification of RHDV
All collected viral samples, either HA positive or negative, were confirmed for the presence of RHDV virus by VP60 gene based RT-PCR assay. Ten viral samples out of 11 were RT-PCR positive for RHVD (Fig. 1).
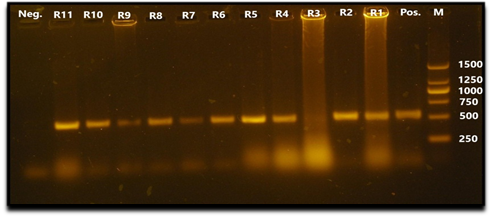
Fig. 1 Agarose gel electrophoresis PCR assay showing amplification of 538 bp fragment of VP60 gene. Lane 1: negative control. Lanes 2,3,4,5,6,7,8,9,11,12: samples R11, R10, R9, R8, R7, R6, R5, R4, R2, R1 (positives). Lane 10: sample R3 (negative). Lane 13: positive control. Lane M: 250 bp DNA marker (Thermo fisher scientific Inc.).
Virus inoculation in susceptible rabbit
The experimentally inoculated rabbits with the first passage of viral samples had the same clinical picture and post mortem (PM) lesions of natural RHDV infection; deaths occurred 3-5 days post infection. The control non-infected rabbits kept alive, without any symptoms. The liver suspension harvested from the inoculated rabbits was confirmed to contain RHDV by microtiter plate HA test. Ten out of 11 viral samples, after first passage on susceptible rabbits, were positive with HA titers ranged from 9-13 log2, as shown in Table 1.
Sequencing and phylogenetic analysis
Phylogenetic analysis of VP60 gene revealed that two isolates (R5 and R6) were clustered into RHDV2 strains group when compared to RHDV2 strains available on gene bank.
The amino acid identity percent of the two isolates was 95.9-97.9% compared to available RHDV2 strains on gene bank. On the other side, these isolates showed 99.2 % amino acid identity percent between them, as shown in Figure 2.
Evaluation of two prepared vaccines containing rabbit hemorrhagic disease virus 2 adjuvanted with aluminum hydroxide gel or Montanide ISA 206
Assessment on virus inactivation
All rabbits injected with formalin-treated virus kept alive without any clinical sign. The inactivated virus titer was 211 HAU by microplate HA test.
Sterility and safety
The sterility test showed the prepared homologous vaccines were free from bacterial and fungal contamination. Regarding safety test, the prepared vaccines were found safe. No clinical symptoms appeared in seronegative susceptible rabbits, after S/C inoculation with double field dose.
Potency
Hemagglutination inhibition test
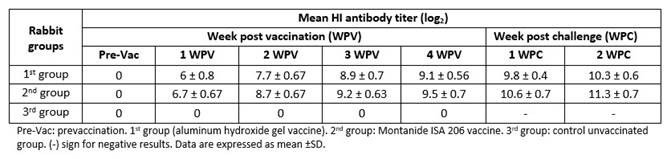
The mean antibody titers against RHDV of post vaccination serum samples of rabbits showed mean HI antibody titers for 2nd group (Montanide ISA 206) slightly higher than for 1st group (adjuvant aluminum hydroxide gel), at one-week post vaccination (WPV). Mean HI antibody titer increased gradually until reach its peak at fourth WPV (9.1 and 9.5 log2 for 1st and 2nd groups respectively). These results revealed that the oil adjuvanted vaccine elicited an earlier and higher humoral immunity than aluminum hydroxide gel adjuvanted vaccine. There were significant differences at first and second WPV (p˂0.05) but no significant differences at third and fourth WPV. The RHDV antibody titers of rabbits that survived at fourth WPV were monitored, starting from first to second weeks post challenge. HI test showed an earlier induction of anti-RHDV antibodies in vaccinated rabbits with inactivated oil vaccine and a higher humoral immune response against RHDV2 infection, when compared with inactivated gel vaccine, as shown in Table 2.
Protection
It was found the Montanide oil RHDV2 vaccine conferred better protection (%) to immunized rabbits than the aluminum hydroxide gel RHDV2 vaccine. All the rabbits in the 3rd group died and showed clinical signs and PM lesions of RHD, beginning 2 days post challenge, with variable severity, as shown in Table 3.
Discussion
RHDV is a calicivirus that causes RHD, characterized by necrotizing hepatitis with a high case fatality rate in rabbits.14 Vaccination is considered the most effective approach to reduce morbidity and mortality associated with epidemics.7 Despite control measures, the virus still has managed to spread widely within a short period of time after emerging of new RHDV2 strains in Egypt during 2018.8 Then, updating the current situation is very important to resolve this problem and establish the new strategy to control that disease. In this study, RHDV was diagnosed from 11 infected farms from Qalubia governorate during 2019. Isolation and characterization of RHDV2 from infected samples and preparation of inactivated homologous RHDV2 vaccine was done. Firstly, liver samples were tested by HA test at 4°C.5 Ten out of 11 samples were positive, with HA titers ranging from 28 to 213 which corroborates that RHDV isolates agglutinates human RBC of type “O”, as was reported by Le Gall-Reculé et al.15 HA test cannot rely on diagnosis or typing of RHDV field isolates in accordance to Abd El-Moaty et al.16 So, the application of RT-PCR assay was more sensitive, specific and rapid for the diagnosis and subtyping of RHVD. The VP60 based RT-PCR was applied for detection of RHDV specific nucleic acid11 and it was revealed that 10 out of 11 viral samples were positive. The positive PCR viral samples were injected in experimental rabbits for propagation and isolation; afterwards HA test was applied for first passage samples. Ten samples were positive with HA titers ranging from 29 to 213. Based on the phylogenetic analysis of VP60 gene, the RHDV strains isolated in this study are clustered with Egyptian RHDV2 strain, previously identified by Hameida et al.8 The current isolates (A/Qalubia/2019 and B/Qalubia/2019) showed 99.2% identity between them, while they showed a variability of 2.1-2.5% with respect to the first Egyptian strain of RHDV2 isolated in 2018, on both nucleotide and amino acid sequence. These results agreed with the genetic diversity previously reported by Hemeida et al,8) who found a nucleotide variation among RHDV2 isolates ranged from 1.3-7.3%. These variations between Egyptian RHDV2 indicated this virus was rapidly evolved and mutated since it was identified.
There is no cross-protection immunity between RHDVa and RHDV2. That’s why, OIE 20185 advised to use homologous vaccine containing the strain that was identified during the outbreak. In the present study, inactivated homologues vaccine against RHDV2 was prepared using Montanide ISA 206 oil or aluminum hydroxide gel adjuvants. The finding proved the two prepared vaccine were sterile and safe, in accordance with the recommendations of OIE, 2018.5 Evaluation of inactivated vaccine potency is mainly based on vaccination challenge and vaccination serological response methods, in the vaccine target host. Individual blood samples were collected weekly from all groups and RHDV HI antibodies were measured using HI test.5) Obtained results showed the induction of a higher serological response in the rabbits inoculated with the RHD vaccine adjuvanted with Montanide ISA 206, according to RHDV HI antibody levels in their sera (6.7, 8.7, 9.2 and 9.5 log2) compared to the mean HI antibody titer of the sera of the immunized rabbits with vaccine adjuvanted with aluminum hydroxide gel that reached to 6, 7.7, 8.9 and 9.1 log2 at first, second, third and fourth WPV respectively. In parallel, it was presented a significant difference between 1st and 2nd group at first and second WPV. So, the earlier and better immune response was demonstrated in vaccine adjuvanted with Montanide ISA oil from first WPV until the end of experiment. These results agreed with, Hansen et al,17 who said that the adsorption of antigen onto aluminum containing adjuvants may significantly decrease the amount of eluted antigen from aluminum salts leading to weak antibody response. Challenged vaccinated rabbits with oil adjuvanted vaccine had higher immune response to challenged rabbit group inoculated with aluminum hydroxide gel vaccine. After 7, 14, 21 and 28 days post vaccination, ten rabbits from each group were challenged intramuscularly at a dose 1mL/rabbit containing 100 LD50 of homologous RHDV2 virus. Protection (%) was 60% and 70% for 1st and 2nd groups respectively at first WPV; these results agreed with Hogenesch,18 who reported that the aluminum hydroxide gel depends on depot effect to stimulate the immune response; it was taken more than one week to come up with protective immunity against highly mutated RHDV-2. The protection was increased to 100% in oil adjuvant vaccine, while aluminum hydroxide gel vaccine was given 90% protection against RHDV2 at third and fourth WPV, which was identical with that recorded by Shevchenko, 1994.19 The prepared vaccines were potent according to OIE, 2018 5 who said that the vaccine should give not less than 90% protection percent to be valid. These results were in agreement with Amal et al,20 who recorded that rabbits developed full protection against RHDV infection 3 weeks after the administration of one dose of inactivated RHDV vaccine.
Therefore, a continuous monitoring and molecular characterization of RHDV strains present in Egypt is recommended, to update the vaccine strain and avoid vaccination failure. The vaccine formulation should contain Montanide ISA 206 as an adjuvant instead of aluminum hydroxide gel.