Introduction
Newcastle disease (ND) is a viral disease of poultry caused by Newcastle disease virus (NDV), a single-stranded RNA avian paramyxovirus type 1. The disease is present worldwide and infects most bird species, causing huge losses in the poultry sector. In developing countries, outbreaks of ND have occurred in many areas, resulting in severe economic and commercial losses. It is an endemic disease in Egypt.1)
The primary NDV control strategies depend mainly on vaccination with live attenuated or inactivated vaccines.2 In United States, during the outbreak of California in 2002-2003, about 2,500 premises (4 million birds) were depopulated. Losses were estimated at $162 US million. In 2008, the World Organization for Animal Health (OIE) considered ND with certain virulence criteria, a notifiable disease.3
Mycoplasma gallisepticum (MG) is a bacterial pathogen which causes chronic respiratory disease (CRD) with economic losses.4) It was listed by the OIE as a primary cause of CRD of poultry.3 The infection usually produces mild symptoms with the ability to synergize with other avian respiratory agents such as infectious bronchitis virus, NDV and Escherichia coli, therefore mycoplasmosis is considered an economically important disease.5 Poultry producers depend mainly on vaccination and biosecurity to manage mycoplasmosis.6
MG induces immunosuppressive effects by damaging the immune system and affecting B and T cells development, leading to an impairment of chicken immune system. In turn, it leads to down-regulation of the post-vaccination immune response and consequently results in limited development of protection. Earlier reports demonstrated the immunosuppressive effect of MG in chickens vaccinated with NDV vaccine. This was confirmed by the reduction of hemagglutination inhibition (HI) titer and IgG antibody titers against NDV,7) as well as delayed cytokine response initiation. Additionally, there is a lack of information concerning the immune response to mixed MG and NDV vaccines in Egypt. Studies are needed to evidence the adverse effects of MG vaccination on the immune system at the time of ND vaccination.
The present study was conducted to evaluate the most protective and effective vaccination program against ND and GM vaccines, in order to contribute to the optimization of vaccination programs to achieve the best flock immune status.
Material and Methods
Vaccines
Three commercial vaccines were used in the current study: a live attenuated MG vaccine, an inactivated MG vaccine and an inactivated ND vaccine.
Virus
A virulent NDV local isolate type (genotype 7): 7 NDV-B7-RLQP-CH-EG-12, accession No KM288609 was supplied by the Central Laboratory for Evaluation of Veterinary Biologics (CLEVB), Abbasia, Cairo, Egypt which has been routinely used at CLEVB for challenge testing.
Specific pathogen free chickens and eggs
Three hundred and twenty, 5-weeks old specific pathogen free (SPF) chickens and 9 days old embryonated chicken eggs (ECE) were obtained from the SPF Egg Production Farm, Koum Osheim, El-Fayoum, Egypt.
Blood samples were collected from all groups prior to vaccination to assure its freedom of MG and NDV, to be used as negative control for further investigations.
Experimental design
Eight groups of SPF chickens, 40 chickens per each, were vaccinated and challenged at the following time intervals (Table 1).
Table 1 Vaccination-challenge groups design.
| Age of Vaccination | ||||
| Group | 5 weeks | 8 weeks | 12 weeks | NDV challenge age |
| G1 | MG live attenuated | MG inactivated | 12 weeks | |
| G2 | MG live attenuated | MG inactivated | ND inactivated | 16 weeks |
| G3 | MG live attenuated | 9 weeks | ||
| G4 | MG live attenuated | ND inactivated | 12 weeks | |
| G5 | MG inactivated | 12 weeks | ||
| G6 | MG inactivated | ND inactivated | 16 weeks | |
| G7 | ND inactivated | 9 weeks | ||
| G8 | Control | 16 weeks |
Vaccination and boosting
As shown in Table 1, at 5 weeks old, SPF chicks in G1, G2, G3 and G4 were immunized with live attenuated MG vaccines and G7, with inactivated ND. Three weeks later (8 weeks old), G1 and G2 were boosted with inactivated MG; G4, with inactivated ND and G5 and G6 were vaccinated with inactivated MG. At 12 weeks old, G2 and G6 were vaccinated with inactivated ND. All vaccines were administered by the recommended route (subcutaneous) and dose (0.5mL).
Serum plate agglutination
Blood samples were collected on 28th day after last immunization for serological testing by serum plate agglutination (SPA) test to assure formation of protective antibody against MG.3
Hemagglutination inhibition test
The collected serum samples of all experimental groups were also tested to determine the NDV antibody titer of each vaccinated group by HI test as mentioned in OIE.8 Two fold serial dilutions of serum samples were applied from 1/2 to 1/2048 against 4 HA units of ND antigen 106 EID50/0.1 mL by HA test.8 The geometric mean of ND HI antibody titer was calculated.8,9 The mean HI titer has to be not less than 6 1og2.
Challenge test
Ten birds from each group were challenged (at different weeks according to the group, Table 1) with 106 LD50 NDV(7 NDV-B7-RLQP-CH-EG-12, accession No KM 288609), 1 mL/ bird, and were inspected for further 6 days for clinical signs and mortalities.8,10 Virus shedding post challenge was shown by tracheal swabs collected on days 1, 3 and 5 from live birds.11 All NDV swabs were titrated using 9 day old SPF ECE;12 virus shedding was calculated using Kärber method.9 At the end of observation days, live birds with moderate to severe signs were humanly euthanized for detection of ND post-mortem gross lesions.13 NDV protection percentage has to be 90% or higher.
Results
Serum plate agglutination of Mycoplasma gallisepticum vaccinated groups
All sera from the MG vaccinated groups showed positive results by serum plate agglutination test.
Hemagglutination inhibition titers of Newcastle disease vaccinated groups
The mean HI titer was calculated for all experiment groups (G1-G8) after blood samples were collected separately from each vaccinated group before the challenge test. The G7 immunized with ND vaccine (at 5 weeks) recorded the best protective ND antibody titer (7 log2). Also, G2 recorded a marginal satisfactory antibody titer (6 log2) after vaccination with the three tested vaccines. The remaining groups revealed unsatisfactory titers ranging from 0-5 (Table 2).
Post challenge protection rates
The protection level was evaluated according to the percentage of mortality, morbidity and virus shedding after inoculation of tracheal swabs into 9-day-old SPF ECE. The groups vaccinated with ND showed highest protection rates as follow: 90%, 80%, 70% and 100% for groups G2, G4, G6 and G7, respectively. Only G2 and G7 were considered protected (Table 3).
Table 3 Protection percentages against virulent NDV post challenge.
| n= 10 | |||
| Groups | Positive | Negative | Protection (%) |
| G1 | 8 | 2 | 20 |
| G2 | 1 | 9 | 90 |
| G3 | 8 | 2 | 20 |
| G4 | 2 | 8 | 80 |
| G5 | 10 | 0 | 0 |
| G6 | 3 | 7 | 70 |
| G7 | 0 | 10 | 100 |
| G8 | 10 | 0 | 0 |
Groups G1, G3, G5 and G8 showed typical clinical signs of NDV, mainly nervous manifestations with nasal and ocular discharge. At post mortem (PM) inspection, the same groups showed characteristics of NDV infection, such as punctate hemorrhages in the proventriculus and cecal tonsils, with congested trachea and catarrhal exudates (Fig. 1).
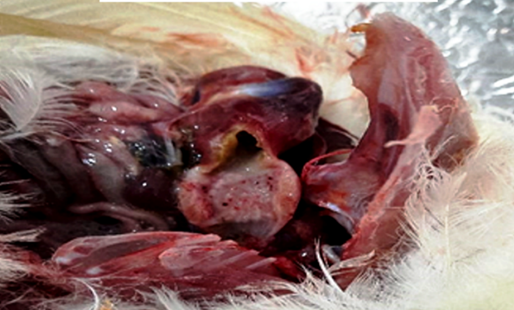
Fig. 1 Post challenge lesion, post mortem inspection, showing pin pointed hemorrhages at the proventriculus.
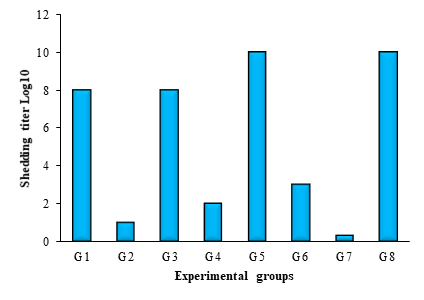
The results of NDV shedding post challenge were consistent with mortality and morbidity rates (Fig. 2). Groups immunized with ND vaccines (G2, G4, G6 and G7) showed low or no virus shedding post challenge.
Discussion
Over the past years, studies have been conducted on immunosuppressive effect of bacteria at the time of ND, which may render vaccine efficacy by affecting the immune response against ND vaccine.14 The current study evaluates the impact of using MG vaccines for chicken immunization before vaccination with ND vaccine, for this purpose, the humoral immune response by detection of antibodies anti-hemagglutinin protein using HI test and the ND vaccine protective efficacy after challenge test were evaluated.15
The antibody titer of the ND vaccinated groups is directly proportional to the immunogen retention time. The present study showed that the humoral immune response against NDV decreased in all MG vaccination models. Low titers of antibodies against NDV in birds vaccinated with either live attenuated or inactivated MG confirmed its immunosuppressive effect.16
The present work also studied the impact of using live attenuated and inactivated MG vaccination on NDV experimental infection. The immune response of all vaccinated groups was evaluated; sera from groups G2, G4, G6 and G7 were especially considered, since these chickens were vaccinated with NDV inactivated vaccine, in addition to different MG vaccines in several schedules.
Groups vaccinated with inactivated or live attenuated MG vaccines and ND vaccine revealed lower NDV antibody titers compared to the group immunized with ND vaccine only. This finding may be explained by the ability of the ND vaccine to induce specific and non-specific immune responses against NDV, which corresponds to the development of protective NDV antibody titers when the ND vaccine was used. Also, an adequate amount of non-specific factors, such as cytokines like interferon gamma, which activate the production of antigen-stimulated B lymphocytes, cytotoxic T lymphocytes, macrophages and natural killer lymphocytes, could be produced.17
On the other hand, groups vaccinated with inactivated or live attenuated MG vaccines before ND vaccination resulted in a marginal NDV antibody titer. This could be attributed to defective B-lymphocyte production in the live attenuated MG vaccinated group.18) In addition, IgM to IgG switching could not have occurred when the inactivated MG vaccine was administered, but could be possible when the live vaccine was used. Furthermore, interferon gamma may not be efficiently produced, which in turn leads to inadequate macrophage activation.17
Mycoplasma vaccines may induce an immunosuppressive effect by affecting B and T cells progress, leading to a drastic impairment of the immune system, accompanied by down regulation of post-vaccinal immune response to ND and the development of a very limited post-vaccination protection against ND. These findings were evidenced by the low level of antibody titers under the effect of MG vaccination.14
The current work revealed that the level of humoral immune response to NDV was decreased with both MG vaccines. The low NDV antibody titers in groups vaccinated with MG and ND vaccines confirmed to be immunosuppressed. This immunosuppression led to a negative impact on ND vaccination. This information can be a guidance when design an appropriate vaccination strategy for the prevention and control of NDV.14
Both live attenuated and inactivated MG vaccines produced an adverse effect on ND vaccine. Unsatisfactory HI titers suggest its negative impact.19 Unfortunately, there is no evidence of immunomodulatory effect of MG vaccination before NDV vaccination.
Additionally, a challenge with virulent NDV was applied to measure the protective immunity induced against NDV. It is evident that the mortality rates of birds vaccinated only with ND vaccine were very low after challenge. Only birds from G2 and G7 were protected (90% and 100%, respectively) when protection percentages and shedding titers were calculated. Groups vaccinated with MG live attenuated/ND vaccines (G4) or MG inactivated /ND vaccines (G6) showed an impotent protection percentage, but still better than ND non-vaccinated groups (G1, G3, G5). This finding supports previous reports of the negative effect of MG vaccines on the inactivated ND vaccine, since vaccinating only with the ND vaccine was the best variant of those studied in the experiment, the birds were able to persist the challenge without mortality, PM lesions, shedding or clinical signs.19
On the other hand, the lower protection levels demonstrated insufficient immunity to protect chickens against NDV, which can be attributed to the immunosuppressive effect of using MG vaccines before inactivated ND vaccination.14