Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.26 n.1 La Habana ene.-mar. 2009
RESEARCH
Epidermal growth factor in the treatment of radiogenic proctitis
Factor de crecimiento epidérmico en el tratamiento de la proctitis radiógena
Nery González1, Francisco Hernández-Bernal2, Tania González2, Josefina Lugo1, Juan J Lence1, Maria A Pascual3, Pedro López-Saura2
1Proctology Department, National Oncology Institute, Street 29 corner F, Vedado, CP 10400, Havana, Cuba
2Clinical Trials Division, Center for Biological Research, Street 134 / 23 and 25, Playa, PO Box 6332, Havana, Cuba
3National Center for the Coordination of Clinical Trials, Street 200 / 19 and 21, Allotment Atabey, Playa, CP 11600, Havana, Cuba
ABSTRACT
Radiogenic proctitis is a frequent complication of therapeutic radiation at the pelvic region. The aim of this study was to determine the efficacy of the epidermal growth factor (EGF) in treating patients with proctitis as a complication of radiotherapy of gynecological tumors. A phase II, placebo-controlled, randomized, double blind clinical trial was carried out. The treatment groups were: A) a solution of human recombinant epidermal growth factor (10 µg/ ml) in carboxymethyl cellulose or B) placebo (carboxymethylcellulose solution alone); the treatment was administered as a retention enema (20 mL) twice a day after bowel movement for 6 months. We included women of 18-75 years of age with proctitis as a complication of an ionizing radiation treatment of gynecological malignant tumors, confirmed by endoscopy, who gave their consent to participate. Thirty-seven patients were included. The basic demographic variables and were homogeneous among groups, which were comparable. There were more responses in group A (EGF) than in the control (placebo), but the difference was not statistically significant. Considering only those patients who completed the treatment, the difference in response was greater with EGF (86% vs 60%). The symptoms disappeared more rapidly in the group treatedwith EGF (p=0.027 and p=0.016, for bleeding and tenesmus, respectively). The product under study was well tolerated. Although there are signs of improvement in some symptoms, the sample was too small to demonstrate the effectiveness of Hebervis in the treatment of proctitis.
Keywords: EGF, proctitis, radiotherapy, clinical trial.
RESUMEN
La proctitis radiógena es una complicación frecuente de la terapia radiante en la región pélvica. El objetivo de este estudio fue determinar la eficacia del Factor de Crecimiento Epidérmico (EGF) en el tratamiento de pacientes con proctitis como una complicación de la radioterapia de tumores ginecológicos. Se ejecutó un ensayo clínico fase II, controlado con placebo, aleatorizado y doble ciegas. Los grupos de tratamiento fueron: A) Solución viscosa de carboximelticelulosa con aditivo de EGF (10 µg/mL) y B) Placebo (solución viscosa de carboximelticelulosa); el tratamiento se administró en forma de enemas a retener (20 mL) dos veces al día, después de la evacuación intestinal, durante 6 meses. Se incluyeron mujeres con edades comprendidas entre 18-75 años, con proctitis como una complicación de la radiación ionizante del tratamiento de tumores malignos ginecológicos, confirmados por endoscopia, que dieron su consentimiento de participación. Treinta y siete pacientes fueron incluidas. Las variables demográficas y de base fueron homogéneas entre los grupos, por lo que fueron comparables. Hubo más respuestas en el grupo A (EGF) que en el control (placebo), pero la diferencia no fue estadísticamente significativa. Considerando solamente las pacientes que completaron el tratamiento, la diferencia en la respuesta fue mayor (86% vs 60%). Los síntomas desaparecieron más rápidamente en el grupo tratado con EGF (p=0.027 y p=0.016, para el sangrado y el tenesmo, respectivamente). El producto en estudio fue bien tolerado. Aunque hay indicios de mejoría de algunos síntomas, la muestra fue insuficiente para demostrar la eficacia del Hebervis en la curación de la proctitis.
Palabras clave: EGF, proctitis, radioterapia, ensayo clínico.
INTRODUCTION
Pelvic radiotherapy (RT) may be complicated by radiation proctitis. This is a distinct pathological process confined to the lower 25 cm of the large intestine caused by damage to the rectal mucosa. Acute radiation proctitis can develop during, or shortly after, a course of radiation therapy. It is expressed as diarrhoea and tenesmus and usually during a short term. Chronic radiation proctitis (CRP) occurs after 3 months post radiotherapy and is characterized by the painless passage of blood by the rectum (clots or streaking of the stool), rectal mucous discharge, frequent bowel movements and rectal pain. Less commonly observed are, bowel obstruction, fistulae, bowel perforation, and severe rectal bleeding, which may require blood transfusions (1-7).
Acute radiation injury results from the death of mitotically active intestinal crypt cells, whereas chronic radiation injury is the result of progressive endarteritis leading to hypovascular, hypocellular and hypoxic tissue (7-8). CRP is associated with prominent structural changes including mucosal atrophy, intestinal wall fibrosis and vascular sclerosis. CRP can develop either as a consequence of an unhealed acute rectal injury or after a latent period of at least 90 days (8). Denham et al. reported that patients who experienced acute proctitis were at least two times more likely to develop more severe late RTOG/EORTC grades (Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer) than patients who did not (9).
About 85% of the cases observed within the first 2 years after RT. Although the true incidence is unknown, estimates from retrospective data suggest that between 2 and 20% of the patients receivinv radical pelvic irradiation may be at risk of developing CRP (10). This risk will be influenced by both the treatment (e.g.) dose per fraction, total dose and technique) and the characteristics of the patient (e.g, diabetes mellitus, inflammatory bowel disease, hypertension or peripheral vascular disease)(10).
Treatments for CRP are not universally successful. Current modalities include pharmacological agents such as oral and rectally administered steroids, 5-amino salicylates, sucralfate, short chain fatty acid enemas, intrarectal amifostine suspension, oral metronidazole, oral vitamins E and C (2, 8, 11-22). Local haemostatic treatments include topical formalin, yttriumaluminumgarnet laser and surgical intervention, and the use of a defunctioning colostomy for severe cases (3, 23-29). Hyperbaric oxygen therapy has previously been described as a non-invasive therapeutic option for the treatment of radiation proctitis (2-3, 30-32).
Alert et al. reported a CRP incidence of 38% for irradiated patients with uterocervical cancer in Cuba (33). In a previous report, evidence was found on the efficacy of using EGF in the treatment of radiation proctitis (34). The above facts led us to evaluate the effectiveness of human recombinant epidermal growth factor (hrEGF) in patients affected by proctitis resulting from ionizing radiation treatments.
MATERIAL AND METHODS
Trial design and patient selection
A phase II, placebo-controlled, randomised, and double blind study was conducted. Patients were randomly distributed in two groups to receive: A) a solution of human recombinant epidermal growth factor (10 µg/mL) in carboxymethyl cellulose or B) the placebo (carboxymethylcellulose solution alone). The trial was performed at the Proctology Department of the National Oncology Institute, Havana. The protocol was approved by the Ethics Committee of the participating hospital and by the Cuban regulatory authority.
Eigtheen to seventy-five-years old women with proctitis as a complication of an ionizing radiation treatment of malignant gynaecologic tumors were confirmed as eligible by endoscopy. All patients gave their written, informed consent to participate in the trial. Patients who could not attend the outpatient follow-up and treatment as required in the protocol were excluded.
Proctitis diagnosis was done according to Sherman (35), who established four grades of the disease, depending on endoscopic characteristics: Grade I- hyperemia, mild oedema, changes in the vascular pattern, mostly with telangectasias; Grade II- the presence of some or all the signs in grade I are enhanced, and mild bleeding can appear, with the presence of typicall small whitened ulcerations; Grade III- stenosis and well established ulcers are added to the above characteristics; and Grade IV- intense reddening and oedema of the mucosa, important stenosis with large and deep ulcers, and moderate bleeding; a rectal-vaginal or rectal-cystic fistula can be present.
Products, treatment and patient management
Hr-EGF (52:51 aminoacids ratio of 60:40%; >95% pure) was produced in Saccharomyces cerevisiae at the Center for Genetic Engineering and Biotechnology (Havana), and formulated as 10 µg/mL in an aqueous solution containing 1% carboxymethylcellulose, 2.2%v/v ethanol, 0.25% methylparabene, 0.02% propylparabene, and 10% v/v glycerine. The placebo solution had the same composition except for the EGF.
All persons included were outpatients. The treatment consisted of 20 mL retention enemas twice daily, after intestinal evacuation during 6 months. The product was instilled with a 20 mL syringe, through a thin rectal tube, which was graduated at 5 and 10 cm from the end. The patient was instructed to introduce the tube up to the 10 cm mark then instillate the first 10 mL of the solution, later withdraw the tube up to the 5 cm mark and instillate the rest of the enema. This covered the entire rectal wall with the solution.
There were no concomitant treatments. Patients had to fill a compliance card each time they received an enema.
The primary variable was the severity of proctitis (grade). It was measured by rectoscopy, before the treatment and at 2, 4, and 6 months. A more frequent evaluation could be done if there was an important improvement or worsening of the symptoms. Safety was also evaluated through rectoscopy, looking for additional rectal mucosa damage. All rectoscopies were done by the principal investigator.
According to the symptoms (bleeding, diarrhoea, tenesmus, and pain) patients were classified as remission, improved, no change and progression.
Statistics
Sample size was calculated assuming a 25% spontaneous remission rate and a 30% increase to be obtained with the experimental treatment, with a 5% type I α error and 10% type II β error. Forty five patients per group were estimated.
Baseline comparisons among groups were done using the chi-square test and ANOVA for discontinuous and continuous variables, respectively. All analyses were done under the intention to treat (ITT) basis. Statistical processing was done using the SPSS 6.0 for Windows Statistical Information Package.
RESULTS
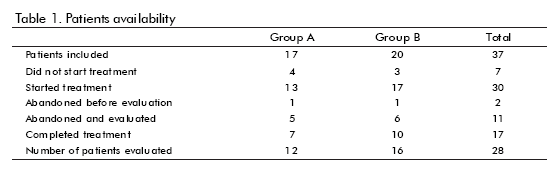
Thirty-seven patients were included. The general trial flow is shown in table 1. Seventeen patients completed the treatment. However, 28 were evaluated under ITT basis. Only patients who did not begin the treatment or abandoned the follow-up were excluded from the evaluation. The reasons for withdrawal are shown in table 2. Most of them were voluntary dropouts.
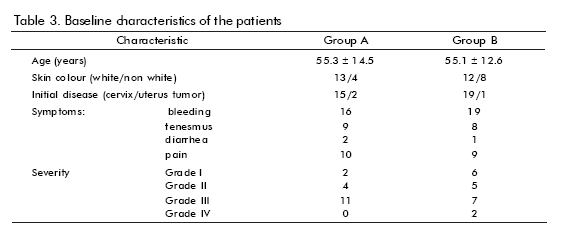
Baseline features are shown in table 3. There were no significant differences between groups concerning age, skin colour, or initial disease (mostly cervix cancer), proctitis severity or clinical features. Smoking or drinking, history of adverse reactions to drugs, bad prognosis factors, type of irradiation received (mostly radium + cobalt), were also analyzed (not shown), without showing any significant difference. Disease-associated anemia was more frequent in group B.
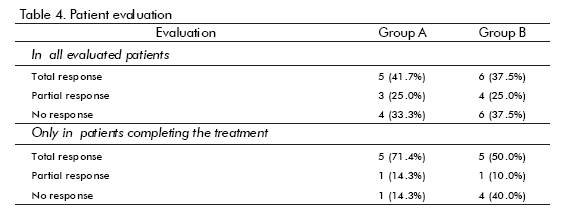
The results of the evaluation of patients are shown in table 4. There were more responses in Group A (hrEGF) than in the controls (67 vs 62%), but the difference was not statistically significant. If only patients who completed treatment are considered, then the difference in response is larger (86% vs 60%), although still not statistically significant. The only hrEGF-treated patient that did not respond had anaemia due to intense bleeding. On the other hand, symptoms disappeared faster in the hrEGF treated group (p = 0.027 for bleeding and p = 0.016 for tenesmus).
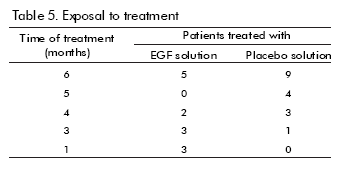
Thirteen patients were exposed to the treatment with the hrEGF solution whereas 17 were in the placebo group (Table 5). Two patients reported adverse reactions: one, who received hrEGF, had pain, bleeding and tenesmus; the other one, from the control group, developed severe anaemia. None of them were considered as treatment-related. There were no significant treatment-related clinical laboratory alterations either.
DISCUSSION
Several studies have revealed that exogenous hrEGF administration can promote intestinal epithelial regeneration (36-40). On the other hand, it has been shown that hrEGF can stimulate recovery in tissue necrosis. Several experiments have also demonstrated that intestinal mucosa regeneration depends on hrEGF incorporation (41).
In a previous pilot study in 14 patients (35) with radiation-derived proctitis, healing or significant improvement was achieved after 4 to 8 months of treatment with hrEGF enemas. Symptoms improved earlier, at 1 to 2 months. In this respect, similar results were obtained in this trial, where there was a significant difference in symptom relief, favourable to the hrEGF treatment.
It was not possible to complete the inclusion of patients. Inclusion rate was too slow, even null for several months. This situation was worse, probably because new irradiation equipment was acquired and radiation iatrogeny became less frequent. In fact, there were only 15 cases of radiation proctitis at the Institute for Oncology, Havana in two years. The fact that the sample size was not fulfilled is the main limitation of this trial. Further studies are required to test the hypothesis that hrEGF can contribute to the treatment of patients with radiation derived proctitis. There is no information in the literature on this respect. Nevertheless, hrEGF has been shown to promote protection or healing of mucosal damage, both experimentally (42) and clinically (43-45) so it is likely that with a larger sample size significant results can be obtained.
There were no violations of the randomization schedule or the blinding. Patient compliance was deficient. Only 46% of the patients completed the treatment schedule. Groups were comparable according to the demographic and baseline characteristics, and variables chosen for evaluation were appropriate. The dose and treatment schedule were based on the previous experience obtained with this product in a small series of patients (34).
Concerning the main outcome variable (mucosal healing), the difference between groups was not significant.
However, it seems that treatment compliance is an important factor that can influence response. In fact, when patients completed the six months of treatment, the advantage of hrEGF therapy was more evident (86% vs 60% response). The patient that did not respond to hrEGF had anaemia due to intense bleeding and required blood transfusion. It is known that deficient oxygenation is an important bad-prognosis factor, concerning wound and mucosal healing.
The treatment was safe and the adverse events that occurred were unlikely to be related to the treatment. Otherwise, they were more probably dependent on proctitis itself. In any case, there was only one adverse event in a hrEGF-treated patient, and no life-threatening events were found at all.
There is no effective treatment for radiation proctitis, which appears mostly in developing countries where radiation techniques are not yet state-of-the-art. In fact the results with other treatments have been very poor and some cases have required colostomy. Therefore, the response evidenced in more than 80% of the patients who completed treatment in this study, although not yet definitive is quite encouraging.
Although there was evidence of certain symptom improvement, the sample was insufficient to demonstrate hrEGF efficacy in radiation proctitis healing. A multicenter clinical trial is recommended.
CONFLICT OF INTERESTS
Authors FHB, TGL and PLS are employees of the Center for Biological Research, which is part of the Center for Genetic Engineering and Biotechnology, Havana, where hr-EGF is produced and the formulation was developed. The other authors have no conflict of interests. The study was financed by Heber Biotec, Havana (products and reagents) and the Ministry of Public Health of Cuba (hospital facilities and general medical care of the patients).
REFERENCES
1. Jones K, Evans AW, Bristow RG, Levin W. Treatment of radiation proctitis with hyperbaric oxygen. Radiother Oncol 2006;78(1):91-4.
2. Denton AS, Andreyev JJ, Forbes A, Maher J. Non surgical interventions for late radiation proctitis in patients who have received radical radiotherapy to the pelvis. Cochrane Database Syst Rev 2002;1:CD003455.
3. Sharma B, Pandey D, Chauhan V, Gupta D, Mokta J, Thakur SS. Radiation Proctitis. JIACM 2005;6(2):146-51.
4. Haddock MG, Sloan JA, Bollinger JW, Soori G, Steen PD, Martenson JA. Patient assessment of bowel function during and after pelvic radiotherapy: results of a prospective phase III North Central Cancer Treatment Group clinical trial. J Clin Oncol 2007;25:1255-9.
5. Goldner G, Tomicek B, Becker G, Geinitz H, Wachter S, Zimmermann F, et al. Proctitis after external-beam radiotherapy for prostate cancer classified by Vienna Rectoscopy Score and correlated with EORTC/ RTOG score for late rectal toxicity: results of a prospective multicenter study of 166 patients. Int J Radiat Oncol Biol Phys 2007;67:78-83.
6. Chun M, Kang S, Kil HJ, Oh YT, Sohn JH, Ryu HS. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Biol Phys 2004;58:98-105.
7. Turina M, Mulhall AM, Mahid SS. Frequency and surgical management of chronic complications related to pelvic radiation. Arch Surg 2008;143:46-52.
8. Hauer-Jensen M, Wang J, Denham JW. Bowel injury: current and evolving management strategies. Semin Radiat Oncol 2003;13:357-71.
9. Denham JW, OBrien PC, Dunstan RH, et al. Is there more than one late radiation proctitis syndrome? Radiother Oncol 1999;51:43-53.
10. Tagkalidis PP, Tjandra JJ. Cronic radiation proctitis. Aust NZ J Surg 2001;71:230-7.
11. OBrien PC, Franklin CI, Dear KB, Hamilton CC, Poulsen M, Joseph DJ, et al. A phase III double-blind randomised study of rectal sucralfate suspension in the prevention of acute radiation proctitis. Radiother Oncol 1997;45:117-23.
12. OBrien PC, Franklin CI, Poulsen MG, Joseph DJ, Spry NS, Denham JW. Acute symptoms, not rectally administered sucralfate, predict for late radiation proctitis: longer term follow-up of a phase III trial transtasman radiation oncology group. Int J Radiat Oncol Biol Phys 2002;54:442-9.
13. Cavcic J, Turcic J, Martinac P, Jelinciæ Z, Zupancic B, Panijan-Pezerovic R, et al. Metronidazole in the treatment of chronic radiation proctitis: clinical trial. Croat Med J 2000;41:314-8.
14. Denton AS, Andreyev HJ, Forbes A, Maher EJ. Systematic review for nonsurgical interventions for the management of late radiation proctitis. Br J Cancer 2002;87:134-43.
15. Gul YA, Prasannan S, Jabar FM, Shaker AR, Moissinac K. Pharmacotherapy for chronic hemorrhagic radiation proctitis. World J Surg 2002;26:1499-502.
16. Kneebone A, Mameghan H, Bolin T, Berry M, Turner S, Kearsley J, et al. The effect of oral sucralfate on the acute proctitis associated with prostate radiotherapy: a double-blind, randomized trial. Int J Radiat Oncol Biol Phys 2001;51:628-35.
17. Melko GP, Turco TF, Phelan TF, Sauers NM. Treatment of radiation-induced proctitis with sucralfate enemas. Ann Pharmacother 1999;33:1274-6.
18. Pinto A, Fidalgo P, Cravo M, Midões J, Chaves P, Rosa J, et al. Short chain fatty acids are effective in short-term treatment of CRP: randomized, double-blind, controlled trial. Dis Colon Rectum 1999;42:788-95.
19. Sanguineti G, Franzone P, Marcenaro M, Foppiano F, Vitale V. Sucralfate versus mesalazine versus hydrocortisone in the prevention of acute radiation proctitis during conformal radiotherapy for prostate carcinoma. A randomized study. Strahlenther Onkol 2003;179:464-70.
20. Kneebone A, Mameghan H, Bolin T, Berry M, Turner S, Kearsley J, et al. Effect of oral sucralfate on late rectal injury asso-ciated with radiotherapy for prostate cancer: A double blind, randomized trial. Int J Radiat Oncol Biol Phys 2004;60:1088-97.
21. Kennedy M, Bruninga K, Mutlu EA, Losurdo J, Choudhary S, Keshavarzian A. Successful and sustained treatment of chronic radiation proctitis with antioxidant vitamins E and C. Am J Gastroenterol 2001;96:1080-4.
22. Singh AK, Menard C, Guion P, Simone NL, Smith S, Crouse NS, et al. Intrarectal amifostine suspension may protect against acute proctitis during radiation therapy for prostate cancer: a pilot study. Int J Radiat Oncol Biol Phys 2006;65:1008-13.
23. Ismail MA, Qureshi MA. Formalin dab for haemorrhagic radiation proctitis. Ann R Coll Surg Engl 20 2;84 (4)(4):263-4.
24. Coyoli-Garcia O, Alvarado-Cerna R, Corona-Bautista A, Pacheco Perez M. The treatment of rectorrhagia secondary to postradiation proctitis with 4% formalin. Gynecol Obstet Mex 1999;67:341-5.
25. Silva RA, Correia AJ, Dias LM, Viana HL, Viana RL. Argon plasma coagulation therapy for haemorrhagic radiation proctosigmoiditis. Gastrointest Endosc 1999;50(2):221-4.
26. Martínez LR, Díaz-Canel FO, Anido EV, Ruiz TJ, García-Menocal HJL. Endoscopy ablation with argon in actinic proctitis. Rev Mex Colo-pro 2007;13(3):95-9.
27. Luna-Perez P, Rodriguez-Ramirez SE. Formalin instillation for refractory radiationinduced hemorrhagic proctitis. J Surg Oncol 2002;80:41-4.
28. De Parades V, Etienney I, Bauer P, Bourguignon J, Meary N, Mory B, et al. Formalin application in the treatment of chronic radiation- induced hemorrhagic proctitis-an effective but notrisk-free procedure: a prospective study of 33 patients. Dis Colon Rectum 2005;48:1535-41.
29. Cullen SN, Frenz M, Mee A. Treatment of haemorrhagic radiation-induced proctopathy using small volume topical formalin instillation. Aliment Pharmacol Ther 2006;23:1575-9.
30. Girnius S, Cersonsky N, Gesell L, Cico S, Barrett W. Treatment of refractory radiationinduced hemorrhagic proctitis with hyperbaric oxygen therapy. Am J Clin Oncol 2006;29:588-92.
31. Mayer R, Klemen H, Quehenberger F. Hyperbaric oxygen: an effective tool to treat radiation morbidity in prostate cancer. Radiother Oncol 2001;61:151.
32. Clarke RE, Tenorio LM, Hussey Jr, Toklu AS, Cone DL, Hinojosa JG, et al. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys 2008;72:134-143.
33. Alert J. Evaluación de la eficiencia de diferentes modelos de fraccionamiento de la dosis de irradiación en los carcinomas de la piel. Rev Cubana Oncol 1987;3:27-31.
34. Lago R, Pascual MA, Barroso MC, Suárez C. Human recombinant Epidermal Growth Factor in the treatment of radiation proctitis. Pilot study. Biotecnol Apl 1993;10(1):10.
35. Sherman LF, Prem KA, Mensheha NM. Factitial proctitis: a re-study at the University of Minnesota. Dis Colon Rectum 1971;14(4):281-5.
36. Thompson JS, Saxena SK, Sharp JG. Effect of urogastrone on intestinal regeneration is dose dependent. Cell Tissue Kinet 1988;21:183-91.
37. Thompson JS, Saxena SK, Greaton C, Schultz G, Sharp JG. The effect of the route of delivery of urogastrone on intestinal regeneration. Surgery 1989;106:45-51.
38. Olsen PS, Poulsen SS, Therkelsen K, Nexo E. Oral administration of synthetic human urogastrone promotes healing of chronic duodenal ulcers in rats. Gastroenterology 1986;90:911-7.
39. Konturek SJ, Dembinski A, Warzecha Z, Brzozowski T, Gregory H. Role of epidermal growth factor in healing of chronic ulcers in rats. Gastroenterology 1988;94:1300-7.
40. Konturek SJ, Brzozowski T, Bielanski W, Warzecha Z, Drozdowicz D. Epidermal growth factor in the gastroprotective and ulcer healing actions of sucralfate in rats. Am J Med 1989;86(Suppl):32-7.
41. Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature 1990;343:82-5.
42. Berlanga J, Prats P, Remirez D, Gonzalez R, Lopez-Saura P, Aguiar J, et al. Prophylactic use of epidermal growth factor reduces ischemia/ reperfusion intestinal damage. Am J Pathol 2002;161(2):373-9.
43. Haedo W, Gonzalez T, Mas JA, Franco S, Gra B, Soto G, et al. Oral human recombinant epidermal growth factor in the treatment of patients with duodenal ulcer. Rev Esp Enferm Dig 1996;88(6):409-18.
44. Palomino A, Hernandez-Bernal F, Haedo W, Franco S, Mas JA, Fernandez JA, et al. A multicenter, randomized, double-blind clinical trial examining the effect of oral human recombinant epidermal growth factor on the healing of duodenal ulcers. Scand J Gastroenterol 2000;35(10):1016-22.
45. Dorticos E, Pavon V, Jaime JC, Reboredo M, Lopez Saura P, Berlanga J, et al. Successful application of epidermal growth factor for treatment of hemorrhagic cystitis after bone marrow transplantation. Bone Marrow Transplant 2003;31(7):615-6.
Received in February 2009.
Accepted for publication in June, 2009.
Francisco Hernández-Bernal. Clinical Trials Division, Center for Biological Research, Street 134 / 23 and 25, Playa, PO Box 6332, Havana, Cuba. E-mail: hernandez.bernal@cigb.edu.cu