Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.26 n.2 La Habana abr.-jun. 2009
REPORT
Physiological study in Saccharomyces cerevisiae for overproduction of a homogeneous human epidermal growth factor molecule*
Jorge Valdés1, Ernesto Mantilla1, Gabriel Márquez1, Regla M Bonilla1, Yanay Proenza1, Michel Díaz1, Saily Martínez1, Willy Frometa1, Yanara Martínez1, Emilio Narciandi2
1Technological Development
2Technology Transfer Department
Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 /158 and 190, Cubanacan, Playa, PO Box 6332, Havana, Cuba
ABSTRACT
The epidermal growth factor (EGF) is a molecule of potent mitogenic activity that accelerates the healing of wounds and ulcers of different tissues in the human body. Over the last years, some institutions of the Havana Western Scientific Pole in Cuba have been developing novel therapeutic products and projects using the human EGF (hEGF) like Heberprot-P® (CIGB) and Cimavax EGF® (CIM). Currently, the growing demand for this molecule has led to strategies being focused on a significant increase in the production capacity of the hEGF. The aim of this work was to increase productivity in the hEGF fermentation process by a combined study of physiological variables, cultivation media and bioreactor operation mode. The influence of culture medium components and the operation during fermentation on the homogeneous production of hEGF and the producer strain growth characteristics were evaluated. A 3.5-fold increase in EGF productivity was obtained by using high cell density fed-batch fermentation process. This fermentation process allowed reaching a cell concentration of 241.6 ± 18.8 g/L (wet mass) and hEGF production of 259.2 ± 37.2 mg/L. This is the first report of hEGF fermentation process expressed in yeasts with productivity of 4.82 ± 0.57 mg/L·h. As part of the analysis, we also discuss the impact of maintaining the highest hEGF expression on productivity in the fermentation process.
INTRODUCTION
A biotechnological production comprises a long and expensive process of research and development, currently estimated around 450-800 million USD. Several basic and key aspects need to be improved to increase benefits and profits, such as producer strain development, culture media design and process parameter optimization able to operate with high production rate and capacity.
The production levels for recombinant proteins depend on the environmental fermentation variables such as: pH, temperature, agitation speed, air flow, and others. There are few reports in the literature of studies analyzing the influence of the culture medium on growth and expression of recombinant proteins (1-5), most of them offering mainly an academic perspective.
In Cuba, the recombinant human epidermal growth factor protein (hEGF) has been obtained extracellularly in cultures of Pichia pastoris and Saccharomyces cerevisiae yeasts, the last one being selected as the appropriate host system for industrial production based on its best results (6). The hEGF is a polypeptide of 53 amino acids with 3 disulphur bridges. It was formerly isolated from submaxillary glands in adult mice and extensively studied as mitogenic agent for epithelial cells (7-10). Its growing demand has led to the design of strategies for significantly increasing its production capacity.
One of the worldwide trends in the developing high productivity of the fermentation processes is the use of fed-batch operation mode at high cell density (over 200 g/L of wet weight or 50 g/L of dry weight) and up to the maximum protein expression potential of the microorganism used (3, 11-13). This has allowed to establish yeast fermentation processes of 6 mg/L·h and 160 mg/L·h for extracellular and intracellular proteins, respectively. More precisely, reports on the expression of hEGF in yeast (6, 14-17) refer to a maximum of 1.98 mg/L·h, also show increased degradation of hEGF at its carboxyl terminus rendering mixed populations of 49- to 52-amino acids molecules (14, 15). Here we describe, for the first time, the over-production of a homogeneous preparation of hEGF by combining the composition of culture medium, process parameters and operation mode for a high productivity of the fermentation process.
RESULTS AND DISCUSSION
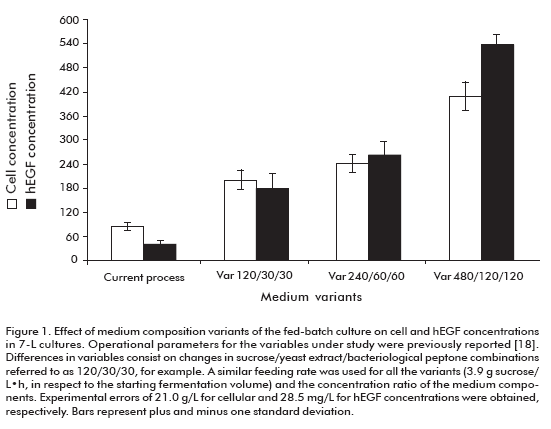
The effect of culture medium concentration (sucrose, bacteriological peptone and yeast extract) on the cell and hEGF concentrations, hEGF productivity and expression are shown in figure 1 and figure 2. The cellular density increased to levels above 200 g/L of wet weight by increasing the concentration of culture media components. The hEGF concentration was increased over 250 mg/L for the variants 240/60/60 (sucrose/yeast extract/bacteriological peptone concentrations, respectively) and 480/120/120, compared to the current variant used as control. Noteworthy, significant differences (95% probability) were observed for both parameters among studied variants.
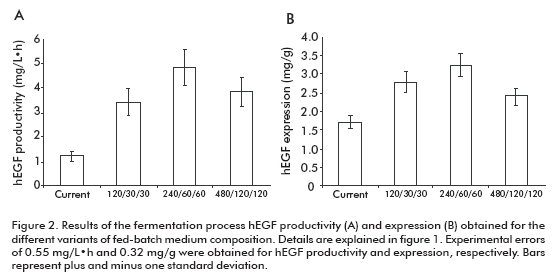
The fermentation productivity of hEGF reached a maximum of 4.82 ± 0.57 mg/L·h for the variant 240/ 60/60, 3.95-fold the variant of the current process (Figure 2A). Significant differences were detected for the 240/60/60 variant compared to all others, except for 480/120/120 variant.
In the case of hEGF expression (Figure 2B), significant differences were detected for the 240/60/60 variant compared to the 480/120/120 and current one, but not for the 120/30/30 variant.
These results allowed us to select the 240/60/60 variant as optimal to establish the fed-batch fermentation process, due to its higher productivity and expression of hEGF. Significantly, this fed-batch culture variant supports the high expression level of hEGF expression of the batch process (18) where culture medium, pH and temperature conditions were optimized with decreased hEGF degradation. The maximum productivity ever reported for hEGF in yeast were of 1.98 mg/L·h in Pichia pastoris (15, 19). Meanwhile, in this work we obtained a 4.82 mg/L·h productivity in S. cerevisiae, 2.4-fold the value previously reported.
The hEGF molecule obtained by using this process was characterized (data not shown), evidencing no degradation at all.
CONCLUSIONS
This study demonstrated a productivity of 4.82 mg/ L·h in the hEGF fermentation process, which is a breakthrough considering the maximum values previously reported in the literature; could thus be achieved by optimizing the physiology of the microorganism, optimizing the concentration of peptone and bacteriological peptone in the culture medium, and establishing the high density (over 200 g/L), fed-batch operation mode during the fermentation process. The use of the 240/60/60 (sucrose/yeast extract/bacteriological peptone concentrations, respectively) variant supported fermentation at the highest expression levels (3.2 mg/g) and an effective control of hEGF degradation in the culture medium (data not shown).
Finally, this fermentation process was successfully scaled up to a 300-L volume and the process change was approved by the Cuban National Regulatory Authority (CECMED). A production capacity, 5-fold higher than the previous one, was achieved in the same industrial facility.
REFERENCES
1. Nowruzi K, Elkamel A, Scharer J, Cossar D, Moo-Young M. Development of a minimal defined medium for recombinant hIL- 3 production by Streptomyces lividans 66. Biotechnol Bioeng 2008;99:214-22.
2. Chen PT and Chao YP. Enhanced production of recombinant nattokinase in Bacillus subtilis by the elimination of limiting factors. Biotechnol Lett 2006;28:1595- 1600.
3. Görgens JF, Passoth V, van Zyl W, Knoetze JH, Hanh-Hägerdal B. Amino acid supplementation, controlled oxygen limitation and sequential double induction improves heterologous xylanase production by Pichia stipitis. FEMS Yeast Res 2005;5:677-83.
4. Wang ZW, Chen Y, Chao YP. Enhancement of recombinant protein production in Escherichia coli by coproduction of aspartase. J Biotechnol 2006;124:403-11.
5. Hahn-Hagerdal B, Karhumaa K, Larsson C, Gorwa-Grauslund M, Gorgens J, van Zyl W. Role of cultivation media in the development yeast strains for large-scale industrial use. Microb Cell Fact 2005;4:1-16.
6. Cinza AM, Quintana M, Lombardero J, Poutou R, Perez E, Perez LC, et al. Establecimiento de un cultivo discontinuo para la producción de factor de crecimiento epidérmico humano en levaduras. Caracterización del producto. Biotecnol Apl 1991;8:166-73.
7. Nakagawa S, Yoshida S, Hirao Y, Kasuga S, Fuwa T. Biological effects of biosynthetic hEGF on the growth of mammalian cells in vitro. Differentiation 1985;29:284-8.
8. Carpenter G and Cohen S. Epidermal growth factor. Ann Rev Biochem 1979;48:193-216.
9. Carpenter G and Cohen S. Epidermal growth factor. J Biol Chem 1990;265:7709-12.
10. Lee DN, Kuo TY, Chen MC, Tang TY, Liu FH, Cheng CF. Expression of porcine Epidermal Growth Factor in Pichia pastoris and its biology activity in early-weaned piglets. Life Sci 2006;78:649-54.
11. Jahic M, Gustavsson M, Jansen AK, Martinelle M, Enfors SO. Analysis and control of proteolysis of a fusion protein in Pichia pastoris fed-batch processes. J Biotechnol 2003;102:45-53.
12. Jahic M, Wallberg F, Bollok M, Garcia P, Enfors SO. Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb Cell Fact 2003;2:6-16.
13. Ohya T, Morita M, Miura M, Kuwae S, Kobayashi K. High-level production of prourokinase-annexin V chimeras in the methylotrophic yeast Pichia pastoris. J Biosci Bioeng 2002;94:467-73.
14. Clare JJ, Romanos MA, Rayment FB, Rowedder JE, Smith MA, Payne MM, et al. Production of mouse epidermal growth factor in yeast: highevel secretion using Pichia pastoris strains containing multiple gene copies. Gene 1991;105:205-12.
15. Clare J, Scorer C, Buckholz R, Romanos M. Expression of EGF and HIV envelope glycoprotein. Methods Mol Biol 1998;103:209-25.
16. Hong S, Moon H, Kim H, Rhee S, Choi E, Kim I. Optimal strategy of pH control in the production of recombinant human epidermal growth factor by Hansenula polymorpha. Proc Biochem 2002;38:487-95.
17. Coppella S, Dhujarti P. A mathematical description of recombinant yeast. Biotechnol Bioeng 1990;35:359-74.
18. Valdés J, Mantilla E, Márquez G, Bonilla R, Lugo V, Pérez M, et al. Improving the expression of Human Epidermal Growth Factor in Saccharomyces cerevisiae by manipulating culture conditions. Biotecnol Apl 2009;26(1):1-9.
19. Hong F, Meinander NQ, Jönsson LJ. Fermentation strategies for improved heterologous expression of laccase in Pichia pastoris. Biotechnol Bioeng 2002;79:438-49.
*This work received the Award of the National Academy of Sciences of Cuba for the year 2008.
Jorge Valdés. Technological Development, Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 /158 and 190, Cubanacan, Playa, PO Box 6332, Havana, Cuba E-mail: jorge.valdes@cigb.edu.cu