Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.26 n.3 La Habana jul.-sep. 2009
REVIEW
Therapies and clinical trials with vaccine candidates against HIV-1
Los tratamientos antirretrovirales y los candidatos vacunales terapéuticos contra el VIH-1
Enrique Iglesias
Centro de Ingeniería Genética y Biotecnología, CIGB Ave. 31 entre 158 y 190, Cubanacán, Playa, CP 10 400, AP 6162, Ciudad de La Habana, Cuba
ABSTRACT
Antiretroviral therapies combining three or more compounds frequently diminish the viral load (VL) in blood to undetectable levels (< 50 copies of RNA/mL), being considered as optimal. In contrast, more than 100 clinical studies with different vaccine candidates have barely achieved modest results and some studies have been discouraging. Therapies are, however, unable to eliminate viral infection. At the same time, they are a threat to the health of patients because of the accumulated toxicity derived from their prolonged use. Many researchers, therefore, believe that an effective (or even partially effective) vaccine might substitute therapies, eliminating the virus or at least controlling the VL through immune-mediated mechanisms. However, immune correlates for protection remain unknown requiring a strategy to evaluate the clinical effectiveness of vaccine candidates. Hence, the experience accumulated with therapies is highly valuable. This paper gives an update on some of the main results of antiretroviral therapies and therapeutic vaccination, giving recommendations in the field of vaccination against HIV-1.
Keywords: antiretroviral treatment, AIDS, therapeutic vaccine, HIV-1.
RESUMEN
Las terapias antirretrovirales que combinan tres o más compuestos, muchas veces reducen la carga viral en sangre a valores no detectables (< 50 copias de ARN/mL), por lo que se consideran muy efectivas. En contraste, más de 100 ensayos clínicos con diferentes candidatos vacunales contra el virus de inmunodeficiencia humana (VIH), solo han alcanzado modestos resultados y algunos estudios han sido decepcionantes. No obstante, las terapias no eliminan la infección viral, por lo que deben administrarse de por vida. Al mismo tiempo, el consumo prolongado eleva la toxicidad a niveles que ponen en riesgo la salud de los pacientes. Por eso, muchos investigadores creen que una vacuna efectiva (incluso parcialmente efectiva) podría sustituir tales terapias. Esa vacuna eliminaría el virus o, al menos, mantendría la carga viral controlada mediante mecanismos inmunes. Sin embargo, los correlatos inmunológicos de protección se desconocen. Por ello, es necesaria una estrategia que permita evaluar la efectividad clínica de los candidatos vacunales. En tal sentido, la experiencia con las terapias antirretrovirales es de gran valor. Se analizaron algunos de los principales resultados de estas terapias y de la vacunación anti-VIH, para emitir recomendaciones en este segundo campo.
Palabras clave: tratamiento antirretroviral, SIDA, vacuna terapéutica, VIH-1.
INTRODUCTION
UNAIDS, the United Nations Program to fight AIDS, has recently estimated that 30 to 36 millions of people in the world are infected with the Human Immunodeficiency Virus (HIV) (1). They estimate that between 1.8 and 2.3 million infected persons have died, and there are 2.2 to 3.2 million new seropositive cases. It is discouraging that all the efforts made to increase the access to antiretroviral therapies (ART), in the year 2007 only reached a quarter of the total number of people infected that year. Therefore, this led to a geometric shortfall every year in the number of people who need antiretroviral treatment and; consequently, most of the seropositives die as a consequence of the impossibility of accessing these therapies. The UNAIDS report expressed that While the percentage of people living with HIV has been stabilized since 2000, the overall number [ ] has steadily increased as new infections occur each year, since HIV treatments extend life as new infections still outnumber AIDS deaths. This explains why the therapeutic scenario becomes more relevant in the context of fighting AIDS and HIV transmission.
Currently, ART are the only effective tool against persistent HIV-1 infection (2). Hence, demands should be maintained to politicians and pharmaceutical companies to make these drugs more accessible to patients. The limited success of these treatments cannot be neglected since they do not eradicate viral infection (3, 4), while causing long-term metabolic disorders (5, 6) making it necessary to interrupt the treatment.
When this time comes, no other therapeutic alternative exists and the disease inevitably progresses, leading to death. An alternative option is the development of immunotherapies to clear the viral infection or to control the infection if the virus cannot be eradicated. Anti- HIV immunotherapies are intended to substitute ART to avoid the negative effects of its continued use.
The aim of this paper is to show the most significant advances in the fields of ART and vaccination against HIV, as starting point to design a strategy for the clinical testing of future vaccine candidates against this virus.
THERAPIES AGAINST HIV
ART against AIDS is perhaps the field of medical practice with the most impressive advances in recent years. Since the discovery of the syndrome in 1981 until the end of the 1980s, the therapy was limited to the specific treatment of the numerous and unusual opportunistic infections commonly causing death. An AIDS diagnosis was equivalent to a death sentence, and certainly, 85% of seropositive persons would die within five years of being diagnosed (7). A few years later, however, together with the enormous number of studies in molecular biology on HIV-1, the first therapeutic compound, zidovudine (AZT), was available. This is a nucleotide analogue inhibiting the reverse transcriptase (NRTI), which was approved in 1987 by the Food and Drug Administration (FDA) of the USA (8). After three years of great expectation the results were discouraging. The CONCORDE study demonstrated that monotherapy results were transient (9). Other nucleoside analogues, such as ddC, ddI and d4T followed a similar fate in the 1990´s. Significant advances were, however, made in treating opportunistic infections after the introduction of co-trimoxazole, pentadimine, gancyclovir, foscarnet, and fluconazole, among other drugs.
At the end of 1995, results from two clinical trials completely changed the conception of ART. Both the DELTA and ACTG175 studies showed that combination therapy with the simultaneous use of two nucleoside analogues was much more effective than monotherapies (significantly increasing survival) (10, 11). Also in 1995, a new class of inhibitors targeting another viral enzyme (protease) was introduced (12, 13). They bind to the active site of this enzyme and prevent the final maturing process of viral particles, making them noninfectious (14, 15). Between December 1995 and March 1996, the FDA readily approved saquinavir, ritonavir and indinavir. In June 1996, at the World AIDS Conference held in Vancouver, the expression highly active antiretroviral therapy was first mentioned, which rapidly spread and was used together with the term tritherapy. The latter refers to a therapy combining three antiviral compounds that markedly decreases mortality (16) and suppresses the viral load (VL) in the plasma (17). During that same month (June 1996) with the approval of nevirapine a new type of antiviral drugs was introduced: the nonnucleosidic reverse transcriptase inhibitors (NNRTI). The NNRTI are structurally different compounds that bind to a region far from the active site of that enzyme but provoking changes on its structure, blocking catalysis. Also in 1996 a new protease inhibitor was licensed: nelfinavir.
Once the tritherapy was introduced and new compounds synthesized, the scenario completely changed. In just four years, from 1994 to 1998, the incidence of AIDS in Europe decreased from almost 31 cases every 100 patients to less than three, and the opportunistic infections practically disappeared (18). Unfortunately, this is not possible in poor countries where the effects of the pandemic are catastrophic because ART are not available, in spite of important agreements made to reduce the prices (19). Besides, the cost of the CD4+ T cell counts test and VL determinations are unaffordable, so the treatment can not be guided based on these criteria.
The success of the tritherapy led to think about the eradication of the virus. But, the existence of viral reservoirs was observed. It was shown that some blood cells are latently infected and low viral replication levels were reported even after tritherapy (20-22); similar to tissues where the virus persists in spite of therapy (23). All attempts to activate these latent viral reservoirs to favor the antiviral activity of the therapies have failed (24, 25). Even worst, it was estimated that more than 70 years of continuous treatment would be required to eradicate these reservoirs (26). This is unlikely since it was evidenced since 1996 that tritherapy lead to many side effects and sometimes fatal metabolic complications, such as lactic acidosis, diabetes mellitus, lipodystrophy, pancreatitis and others (5, 27-29).
Achievements and failures following tritherapy
The aim of antiviral therapies is to prolong life while improving its quality. Its success or failure may be evaluated according to virological, immunological and clinical parameters.
Currently, the methods for VL quantification have a detection limit of 50 copies of viral RNA/mL. For that reason this value of VL was arbitrarily set as the threshold value to assess virological failure. Nevertheless, today it is impossible to assure that 100 or 400 copies of viral RNA/mL can represent a higher risk than 50 copies. After three or four months, ART may reduce VL below the detection level, although it could last somewhat longer in cases with very high VL. But if after six months under treatment the VL persists above detection limits it will be considered virological failure, and physicians would probably consider a change to second line drugs. The decrease of VL in response to tritherapy follows a biphasic pattern.
First, it quickly drops in three to six weeks, slowly decreasing afterwards (30). It has been reported that a VL of 106 viral RNA copies/mL takes approximately four months to decline under undetectable levels (31). Astonishingly, mutant viruses can arise during the first month of the tritherapy, which could limit the effects of some of the drugs in up to 40% of the patients (32). Resistant viruses arising from a previous therapy and the poor adherence to the treatment are the main factors contributing to virological failure for a given therapy (33).
It is common that VL in patients under tritherapy transiently rises up to 500 copies of viral RNA/mL and spontaneously declines to undetectable levels. This can be observed in 20 to 40% of the patients, irrespective of the therapy (34). Moreover, it does not seem to increase the risk of virological failure (35).
The immunological success is normally, and simply defined as the increase in blood CD4+ T cells counts. It is difficult to predict the immunological success for a given therapy because of the patient variability. It was observed that the regeneration capacity of the immune system for naïve CD4+ T cells is heavily impaired in patients over 50 years old (36, 37). Curiously, the kinetics of an increase in CD4+ T cells also follows a biphasic pattern. Similarly during the first 3 to 4 months after the treatment, a 20 cells/μL per month increase can be achieved, followed by a more discrete increase of only 5 cells/μL each month (38).
On the other hand, clinical failure is related to the progression to AIDS and finally death. In the practical sense, clinical success is the most relevant achievement, but it is difficult to assess it and it takes even longer to be unquestionably established. However, several studies have evidenced that clinical success depends in the long-term on virological aspects (39- 41). On the other side, a curious relationship between virological and immunological success has been noticed.
Immunological success (increase in the CD4+ T cell counts) can be achieved at detectable VL (42-44). However, the risk of drop in CD4+ T cells and progression to AIDS is proportional to the increase in VL (45-47). It is noteworthy that this risk starts at certain VL values. In fact, CD4+ T cell counts should not decrease if VL remains below 10 000 RNA copies/mL or 1.5 log under the relatively steady viral set point that has been established during the asymptomatic period (44). Based on all the above, the success of therapies can be assessed based on the virological response. In this sense, the physician has a simple and effective tool to draw conclusions about any therapeutic treatment.
THERAPEUTIC VACCINATION
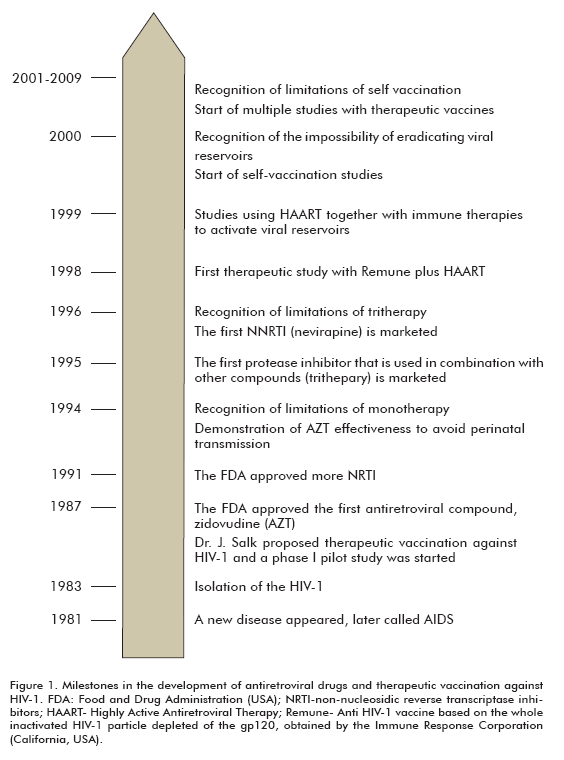
The success/failure ratio of therapeutics against HIV establishes a practical niche for vaccination (Figure 1). This would not be required if there were a therapy able to eradicate the virus, or at least hamper the development of the escape mutant viruses, while stabilizing VL at low levels and keeping it under control in the blood with low toxicity. Many concepts formerly applied to ART have been extrapolated to the therapeutic vaccination field against HIV (such as the success and failure criteria).
Scheduled treatment interruptions and self-vaccination
Tritherapy allows the patients to recover their immunity against several pathogenic agents (48, 49) but the immune response against HIV remains low (50-52). This could come from the very low amount of circulating virus, which is insufficient to activate the immune system (53). That is the reason supporting the research on self-vaccination during scheduled ART interruptions, which transiently increase VL.
Several pilot studies conducted with very few patients, and also in animals, reported encouraging results (54-58), but they did not include a control group. Besides, whichever model was used, the studies only covered subjects with acute infection.
Based on these drawbacks, the SSITT and DART studies were designed and carried out to test the socalled immune and virological benefits of scheduled treatment interruptions at regular time periods in chronic patients, but their results were not too encouraging (59-61). These studies have demonstrated that a partial recovery of the immune response is possible without a detectable control of viral replication (55, 61-64). This could be explained by that fact that self-vaccination only re-stimulates T cell clones which were generated during the process of infection and remain uninfected (65), among other factors. The general consensus establishes that scheduled treatment interruptions in HIV patients at regular time periods can be dangerous in clinical practice.
Another practice consists of the interruption of the tritherapy based on blood T CD4+ cell counts. These counts, together with VL, are the most relevant markers of the progression to AIDS (45-47, 66, 67). In patients with high viral CD4+ T cell counts, tritherapy is interrupted and resumed when this parameter drops below a defined threshold. There are reports of studies using this therapeutic alternative. In general, they have been non-randomized studies with different threshold values and applied in heterogeneous populations. Their authors have concluded that such interruptions are safe and make it possible to reduce the exposure to other therapies (68-71). However, the most relevant results come from the large randomized studies TIBET, STACCATO, ACGT5170, LOTTI, TRIVACAN and SMART. Tritherapy interruptions based on CD4+ T cell counts are safe when VL is under control, according to the results of the TIBET (72), STACCATO (73), ACGT5170 (74) and LOTTI (75) studies, which evaluated 1127 patients under the criterion of CD4+ T cell counts lower than or equal to 350 cells/μL to resume the treatment. In contrast, the TRIVACAN (76) and SMART (77) studies pointed out the opposite direction, but their criteria to resume and stop the therapy were CD4+ T cell counts below 250 and above 350 cells/μL, respectively.
The largest randomized studies up to now, SMART and LOTTI, demand a more detailed analysis. Results of the SMART study, involving 5000 patients in more than 50 countries, have convinced many physicians on how unsafe these interruptions are (78). However, inconsistencies arise from a careful analysis of these results. For example, the increased risk of AIDS and the death of patients could not be related to their CD4+ T cell counts at the beginning of the study. Neither the CD4+ T cell counts nadir, nor the incidence of AIDS were predictive. On the other hand, the risk of becoming ill was low; although of high statistical significance (due to the high statistic power). The LOTTI study, comprising 329 patients, evidenced that neither the risk of progression nor the detection of resistant mutant viruses increase when resuming therapy at CD4+ T cell counts lower than or equal to 350 cells/μL (75). Considering the results of these studies, treatment interruptions based on the T CD4+ cells are not detrimental within certain range of this parameter and under controlled VL, and may be useful to delay or mitigate the appearance of undesired side-effects of therapies (79-80).
Risks associated to the interruption of the treatment against HIV
The treatment interruption studies have shown that VL quickly increases after the interruption. In fact, viremia becomes detectable within 2 or 3 weeks (64, 81, 82); stabilizing at values observed before the treatment (or the viral set point) (83). Similarly, CD4+ T cell counts drop to values closer to the count observed before the treatment. In this case, the lower the CD4+ T cells nadir, the faster the drop in CD4+ T cell counts in the off therapy period, reaching the viral set point in just a few weeks (68).
Symptoms similar to those reported during acute HIV-1 infection commonly appear after therapy interruption, e.g.: lymphadenopathies, fever, asthenia and discomfort (84-86). Thrombocytopenia has been also reported (87), indicating the need for monitoring blood parameters.
The main concern of physicians after treatment interruption is the increase in viruses resistant to these therapies. It is widely considered that the higher the VL and the lower the CD4+ T cell count, the higher the risk of virological failure (the rise of viruses resistant to therapy), immune failure and the appearance of AIDS. This is essentially true, but it should be limited to CD4+ T cell counts below 350 cells/μL (as mentioned in the previous section). Above that threshold and after a single treatment interruption, the risk of virological failure is almost inexistent. One of the first studies evidencing this was the French COMET study (88). Unfortunately, this study was non-randomized, thereby limiting the implications of its results. There have been other cohort studies in patients naïve to therapy that were treated; treatment was interrupted and, once resuming therapy, the rate of virological failure was not higher than that shown by patients treated without interruption (89-92). These studies demonstrate that virological success is hardly compromised when patients have not accumulated previous therapeutic failures. Besides, the risk of developing AIDS is also low (for a single interruption) as shown in the SWISS (93, 94) and AMELIA (71) cohort studies.
Therapeutic vaccines
Therapeutic vaccination was proposed to prevent AIDS by Prof. Jonas Salk in 1987 (95), and the first phase I pilot study was started in that very year, in 25 patients who were immunized with Remune (a whole inactivated HIV-1 virus vaccine depleted of gp120 glycoprotein)(96). This study provided poor but encouraging results in regard to viral control. Nevertheless, the efficacy of this type of treatment was questioned because of the lack of a control group. Other similar studies were carried out using different vaccine candidates, but all of them had the same limitations as the Remune study (reviewed in (97)) and gave no encouraging results. Besides, patients were exposed to an unacceptable risk by facing immunizations when there is not virological control (further discussed in Vaccination-associated risks in seropositive patients). This mostly promoted the interruption of clinical studies evaluating therapeutic vaccines. Fortunately, ART were developed shortly afterwards, with its subsequent and encouraging results in VL. In the 12th World Conference on AIDS, held in Geneva in 1998, a group of researchers showed that a number of seropositive patients subjected to HAART and further immunized with Remune developed a significant proliferative response against p24 (98). This result re-launched the studies using therapeutic vaccines.
Therapeutic vaccination began earlier than the development of the tritherapy and self-vaccination with scheduled treatment interruptions against HIV-1. ART do not eradicate viral reservoirs, having very limited effects when resistant viral mutants appear, and are toxic in the long-term. Because treatment interruptions (self-vaccination) failed to stimulate a protective immunity an intense work has been carried out in recent years to substitute ART by the combination of therapeutic vaccination with tritherapy. In this new scenario, therapeutic vaccination is aimed to stimulate the protective components of antiviral immunity, when ART keeps the viral replication under control. Viral control by immune mechanisms after interrupting therapy should be the final outcome (99). At least two studies in the simian immunodeficiency virus (SIV) model suggest the feasibility of this strategy (100, 101). In fact, many researchers believe that vaccination is the best choice for a long-term virological control after interrupting ART (102-104).
Very recent studies suggest that therapeutic vaccination could help reduce the variability of HIV-1 quasi- species (105) and to expand the functional response of antiviral CD4+ and CD8+ T cells (106-107).
Numerous human clinical trials to enhance cellular immunity with a variety of therapeutic regimes (reviewed in (97)), have evaluated essentially four types of vaccine candidates: 1) subunit vaccine candidates, comprising immunogens based on the viral envelope proteins, Tat toxoid, lipopeptides and p24 virus-like particles, among others; 2) inactivated virus, as Remune; 3) viral vectors, such as ALVAC; and 4) naked DNA. All these strategies have shown that it is possible to stimulate new specificities of the anti-HIV-1 CD4+ and CD8+ T cell responses without producing severe secondary effects (108-117). Unfortunately, it is impossible to predict the efficacy of vaccine candidates from the results of their immunological tests, since the mechanisms mediating protection against HIV-1 remain unknown.
Vaccination-associated risks in seropositive patients
Vaccination can induce a transient peak in the VL of HIV-1 ART naïve seropositive patients, from 2 to 30 times the initial levels. This increase is maximal within 3 to 28 days (with a mean of 13 days), later decreasing up to the initial VL levels in approximately six weeks. This has been demonstrated for other vaccines, such as influenza (118), pneumococci (119, 120) and tetanus toxoid (121). It has been possible to establish that the stimulation of CD4+ T cells by vaccination further increases the viral replication rate through the action of cellular transcription factors (122, 123). It is noteworthy that another series of studies have reported no increases in the VL after vaccination (124-126) that could be explained by the differences in the time points and frequency of laboratory determinations between studies. Nevertheless, and based on such results, the international scientific community has concluded that it is risky to vaccinate HIV-1 seropositive patients who have a high (uncontrolled) VL.
Further studies have been conducted in patients under tritherapy and with controlled VL. Results from Kolber and co-workers, with a small number of patients having a VL lower than 200 copies of RNA/mL and having been vaccinated against influenza, suggest that vaccination induces an increase in plasma virus after two to three weeks, with a relatively high frequency among patients without previous treatment (5/26; 19%) and even higher in those who had previous therapeutic failure (3/8; 38%) (127). Viral mutants were reported in some patients, but the authors did not present results allowing to evaluate the impact of these mutants, and in most of the cases they did not discriminate whether the mutant was present or not before vaccination. The study was further limited by the lack of a negative control group of patients under tritherapy.
Two other studies indicated that vaccination against influenza decreases T CD4+ cell counts (125, 128), although this parameter was only significant in one of them (128).
Another study on vaccination against influenza in a group of HIV-1 seropositive patients with VL lower than 50 copies of viral RNA/mL evidenced an increase of VL in 3 out of 11 patients (27%), which was transient (returning to values prior to vaccination) and never rising above 100 RNA copies/mL (129). It was also observed that the absolute counts of CD4+ T cells and the proportion of naïve cells in those patients were similar to those of the uninfected control groups.
This was not the case for the other groups studied, in which VLs were higher than 50 copies of viral RNA/ mL. The immune response after vaccination was significantly higher in the group showing total suppression of the VL, compared to the groups with a partial control of the VL. Other researchers achieved similar results (130). Vaccination of seropositive patients has been able to establish that it is not enough to reach normal CD4+ T cell counts after ART; instead, a nadir of CD4+ T cell counts higher than 350-400 cells/μL is absolutely required to generate new specificities and to develop a functional T cell response (131).
On the other hand, there are several studies on therapeutic vaccination against HIV-1 in patients under ART showing an increase in VL after vaccination. This increase is infrequent, and never higher than 600 copies of viral RNA/mL, returning to previous levels within few weeks (116, 117). They also showed only mild and transient adverse events associated to vaccination (109-117), even when the vaccine is administered together with immuno-stimulatory molecules (i. e., cytokines and others) (108, 132).
Clinical Trial Designs
The clinical efficacy of any treatment against HIV can be assessed as: 1) the cure or eradication of the virus; 2) a significant increase in progression time to AIDS; or 3) at least an improvement in the quality of life of the patient when goals 1 and 2 are not reached.
However, to achieve statistical significant for the clinical efficacy a large number of patients must be recruited and observed for a long period of time Therefore, the international scientific community and regulatory agencies (such as FDA) have accepted the decrease in plasma VL as a surrogate marker of clinical efficacy (133, 134). This is valid for vaccine candidates that can induce cellular immunity, because their mechanisms of action are analogous to those of ART. Studies on these therapies have shown that VL effectively predicts the rate of progression to AIDS (45-47).
There was also a consensus on the most efficient design of clinical trials. This allows assessing the clinical efficacy after a short observation period (135, 136). A minimal design would comprise a group with HAART and placebo (control group; CG), to be compared to another group receiving HAART and with immunotherapy (study group; SG). After ending the immunotherapy of SG, HAART will be stopped in both groups. VL and CD4+ T lymphocyte counts will be periodically determined to assess the number of patients who need to resume therapy. The latter is required when the CD4+ T cell counts are below 350 cells/μL and if VL is above 50 000 viral RNA copies/mL within 4 weeks after therapy interruption, or if VL is higher than 10 000 copies/mL after 8 weeks. The patients health is not at a high risk during the study when applying these criteria. If the immunotherapy is effective, it would be possible to statistically demonstrate that the off therapy period of patients in the SG was significantly higher than those in the CG. Plasma VL is also a very useful parameter, and the FDA issues an expedite approval for a given drug if it maintains the VL under control for 16 to 24 weeks (133). Other designs have been used, without tritherapy interruption and not evaluating efficacy. Small scale phase I studies can be carried out under the first design, including 20 to 30 patients (10 to 15 on each CG and SG group, respectively), which would permit strategic decisions at low costs. The results would support product development or redirecting research strategies.
A study with the above mentioned design was the QUEST using the vCP1452 (ALVAC) candidate, alone and combined with Remune. This demonstrated that the immunization strategy of ALVAC plus Remune generated an anti-HIV-1 cellular response. In contrast, there was not any improvement in the virological control once HAART was discontinued (137).
The last result halted the research being made with the previous approach. Better results were obtained in other studies, such as the STIR-2102 (108) and the ANRS 093 (114). Together, they demonstrated that the treated groups were benefitted with the therapeutic intervention because patients remain off-therapy a longer period of time compared to the placebo groups.
Obtaining a therapeutic vaccine against AIDS is a priority. Walensky and co-workers have predicted through a mathematical model that even partially effective vaccines could have a considerable impact on the patients quality of life, generating a substantial saving in tritherapy expenses of thousands of dollars per patient (138). Underdeveloped countries need it desperately.
PERSPECTIVES OF VACCINATION AGAINST HIV
The production of a vaccine against HIV has encountered insurmountable drawbacks; among them the lack of an animal model that effectively resembles the human disease and the ignorance of the correlates of protection. There is, however, no absolute need to overcome those obstacles to obtain a vaccine.
It is more difficult to obtain a prophylactic than a therapeutic vaccine. This holds true because the HIV-1 directly attacks the immune system causing overwhelming damages, also infecting immunoprivileged sites. It is possible that a vaccine candidate inducing an immune response that can transiently control VL in a therapeutic scenario would be as effective as a prophylactic vaccine for preventing infection or inducing a slower evolution towards the disease.
Classically, when mentioning studies to obtain a prophylactic vaccine, it is considered to be mandatory to successfully surpass phase I and II clinical trials to reach a phase III stage for testing efficacy. Evidently, these studies demand more funds and numerous human resources, all of which delay the evaluation process of vaccine candidates. Consequently, the urgent need for a vaccine against AIDS and the scarce financial resources of some countries are compromised. There is an international commitment to accelerate the evaluation process of anti-HIV-1 vaccine candidates, with several propositions being discussed (139, 140), and fast-track regulations in many countries (as the CECMED Regulation 27-2000 in Cuba).
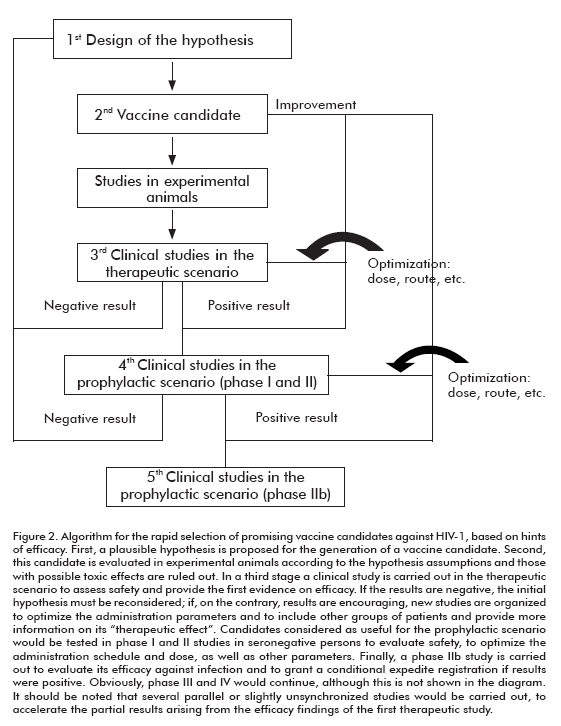
Considering all the above mentioned, an algorithm can be envisaged in which all the vaccine candidates (even the prophylactic ones) and those especially designed to induce an antiviral cellular response could be assessed in a therapeutic scenario for safety and validation of the hypothesis when possible (Figure 2).
Clinical trials will start after the preclinical phase in experimentation animals. These therapeutic vaccination studies, aimed to test safety and to find efficacy evidences, could be run under a design as that proposed in the section of Design of clinical studies, using both CG and SG, treating the seropositive patients having an undetectable VL (or low and controlled VL) under therapy and evaluating the control of the VL once stopping therapy.
Of course, it would be needed the compromise of the community of seropositive patients to support the clinical studies. At the same time, this represents an unprecedented ethical challenge for the scientific community, which should guarantee the maximum safety to the volunteers. The hemochemistry, virological and immunological parameters of the volunteers should be closely monitored during those studies.
After this analysis, it can be considered that studies testing vaccine candidates against AIDS by immunizing patients receiving ART and with undetectable (or low and controlled) VL represents a small risk for the patients health once ART is interrupted. This could be assumed as far as their CD4+ T cell counts never decrease below 350 cells/μL and the VL will be lower than 50 000 copies of viral RNA/mL after the first four weeks of therapy interruption or lower than 10 000 copies after eight weeks. Evidences of efficacy can be found under these conditions and non-promising vaccine candidates can be ruled out quickly.
Evidently, such studies should be unbiased to obtain reliable efficacy results. For this purpose, randomized studies ought to be implemented, also favoring the inclusion of male volunteers in the first stages of clinical research due to the unknown potential teratogenic effects of the vaccine candidate and the adverse reactions been reported more frequently in women (141). Moreover, the thymus regresses with age (142- 144) and markedly in seropositive patients at advanced phases of immune dysfunction (nadir of CD4+ T cell counts below 100 cells/μL)(145), compromising the regenerating capacity for T cells of the immune system. Considering all these, it is recommended to preferably enroll subjects immunocompetent and younger than 45 years, immunocompetence is guaranteed when the nadir of CD4+ T cell counts is higher than or equal to 350 cells/μL (146).
Other criteria should be considered. Significant inter-racial and inter-ethnic differences should be avoided between the control and treatment groups, because interracial differences have been reported in the anti-HIV-1 immune response (147). Following the first efficacy evidences with the vaccine candidate, it would be convenient to replicate the study in female patients, to assess the influence of the genus on the immune response, pharmacodynamic and pharmacokinetic (148). Such studies could be also conducted in young people, children and also in patients coinfected with other viruses of high prevalence in seropositive HIV patients, such as hepatitis B and C viruses.
If there would be a prophylactic vaccine candidate inducing an immune response able to transiently control the VL in the therapeutic scenario, it will have to be evaluated in seronegative patients. The first studies should be phase I for testing safety. Afterwards, some immunization parameters may be optimized, if required prior to testing it in highly exposed seronegative persons to accelerate obtaining efficacy results (phase IIb or proof of concept studies)(139). Those people would be intravenous drug addicts, sex workers and seronegative partners of seropositive patients, among others. Due to the high incidence of HIV infection in these persons a low number of volunteers can be enrolled to achieve a statistical significance. In my opinion, this strategy may guarantee a rational use of human and financial resources and would accelerate the studies aimed to obtain a vaccine against HIV-1.
CONCLUSIONS
AIDS pandemic continue to grow and it is not plausible that an effective vaccine to fight HIV would appear in the upcoming years. Some people are pessimist on the possibility to obtain a vaccine because of the failure of the STEP assay using the Ad5 candidate developed by Merck. However, this failure does not rule out the rationale of vaccines aimed at inducing T cell responses. The facts indicating that it is possible to obtain a vaccine are still valid. The knowledge coming from implementing therapies can be relevant and experience from failures of the vaccine candidates studied so far must also be considered. A greater financial support is required to fund research in the public sector and a more rational use of resources.
Four main reasons justify the algorithm discussed in this paper: 1) the lack of an animal model for the disease; 2) the immune correlates of protection are unknown; 3) AIDS is a disease of global impact; and 4) the algorithm currently used to test vaccine efficacy requires too much time. Therefore, a new algorithm is required to quickly discard any unpromising vaccine candidate and simultaneously provide the necessary elements for the registration and rapid extension of vaccination to those who need it the most.
A proposal has been given that would make possible to rule out an unpromising vaccine candidate in a short period of time of 4 to 5 years. This period is three times shorter than the current working time (11 to 14 years) (140). We hope these ideas are useful not only in the field of vaccination against HIV, but also against pathogens of other diseases complying with the four reasons mentioned above.
ACKNOWLEDGEMENT
The author is very grateful to Dr. Arístides Aguilar for his useful suggestions.
REFERENCES
1. ONUSIDA/OMS. Informe sobre la epidemia mundial de sida. Génova: Programa Conjunto de las Naciones Unidas sobre el VIH/Sida (ONUSIDA) y Organización Mundial de la Salud (OMS), diciembre 2008.
2. Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853-60.
3. Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999;5:512-7.
4. Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA 1997;94:13193-7.
5. Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS (London, England) 1998;12:F51-8.
6. Friis-Moller N, Sabin CA, Weber R, DArminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993-2003.
7. Rothenberg R, Woelfel M, Stoneburner R, Milberg J, Parker R, Truman B. Survival with the acquired immunodeficiency syndrome. Experience with 5833 cases in New York City. N Engl J Med 1987;317:1297-302.
8. Ezzell C. AIDS drug gets green light. Nature 1987;329:751.
9. Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Concorde Coordinating Committee. Lancet 1994; 43:871-81.
10. Delta: a randomised double-blind controlled trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals. Delta Coordinating Committee. Lancet 1996;283-91.
11. Hammer SM, Katzenstein DA, Hughes MD, Gundacker H, Schooley RT, Haubrich RH, et al. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS Clinical Trials Group Study 175 Study Team. N Engl J Med 1996;335:1081-90.
12. Dorsey BD, Levin RB, McDaniel SL, Vacca JP, Guare JP, Darke PL, et al. L-735,524: the design of a potent and orally bioavailable HIV protease inhibitor. J Med Chem 1994;37:3443-51.
13. Vacca JP, Dorsey BD, Schleif WA, Levin RB, McDaniel SL, Darke PL, et al. L-735,524: an orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc Natl Acad Sci USA 1994;91:4096-100.
14. Richman DD. HIV chemotherapy. Nature 2001;410:995-1001.
15. Venaud S, Yahi N, Fehrentz JL, Guettari N, Nisato D, Hirsch I, et al. Inhibition of HIV by an anti-HIV protease synthetic peptide blocks an early step of viral replication. Res Virol 1992;143:311-9.
16. Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997;337:725-33.
17. Hirsch M, Steigbigel R, Staszewski S, Mellors J, Scerpella E, Hirschel B, et al. A randomized, controlled trial of indinavir, zidovudine, and lamivudine in adults with advanced human immunodeficiency virus type 1 infection and prior antiretroviral therapy. J Infect Dis 1999;180:659-65.
18. Mocroft A, Katlama C, Johnson AM, Pradier C, Antunes F, Mulcahy F, et al. AIDS across Europe, 1994-98: the EuroSIDA study. Lancet 2000;356:291-6.
19. Hill A, Wood E. Balancing effectiveness and access to HIV treatment in the developing world. AIDS (London, England) 2007;21:361-3.
20. Pomerantz RJ. Residual HIV-1 infection during antiretroviral therapy: the challenge of viral persistence. AIDS (London, England) 2001;15:1201-11.
21. Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997;278:1295-300.
22. Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997;278:1291-5.
23. Zhang H, Dornadula G, Beumont M, Livornese L Jr, Van Uitert B, Henning K, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med 1998;339:1803-9.
24. Pomerantz RJ. Reservoirs of human immunodeficiency virus type 1: the main obstacles to viral eradication. Clin Infect Dis 2002;34:91-7.
25. Kulkosky J, Nunnari G, Otero M, Calarota S, Dornadula G, Zhang H, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J Infect Dis 2002;186:1403-11.
26. Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003;9:727-8.
27. Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS (London, England) 2000;14:F25-32.
28. Safrin S, Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS (London, England) 1999;13:2493-505.
29. Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 1999;353:2093-9.
30. Wu H, Kuritzkes DR, McClernon DR, Kessler H, Connick E, Landay A, et al. Characterization of viral dynamics in human immunodeficiency virus type 1-infected patients treated with combination antiretroviral therapy: relationships to host factors, cellular restoration, and virologic end points. J Infect Dis 1999;179:799- 807.
31. Rizzardi GP, De Boer RJ, Hoover S, Tambussi G, Chapuis A, Halkic N, et al. Predicting the duration of antiviral treatment needed to suppress plasma HIV-1 RNA. J Clin Invest 2000;105:777-82.
32. Metzner KJ, Allers K, Rauch P, Harrer T. Rapid selection of drug-resistant HIV-1 during the first months of suppressive ART in treatment-naive patients. AIDS (London, England) 2007;21:703-11.
33. Deeks SG. Determinants of virological response to antiretroviral therapy: implications for long-term strategies. Clin Infect Dis 2000;30 Suppl 2:S177-84.
34. Sungkanuparph S, Overton ET, Seyfried W, Groger RK, Fraser VJ, Powderly WG. Intermittent episodes of detectable HIV viremia in patients receiving nonnucleoside reverse-transcriptase inhibitor-based or protease inhibitor-based highly active antiretroviral therapy regimens are equivalent in incidence and prognosis. Clin Infect Dis 2005;41:1326-32.
35. Martínez V, Marcelin AG, Morini JP, Deleuze J, Krivine A, Gorin I, et al. HIV-1 intermittent viraemia in patients treated by non-nucleoside reverse transcriptase inhibitor-based regimen. AIDS (London, England) 2005;19:1065-9.
36. Grabar S, Kousignian I, Sobel A, Le Bras P, Gasnault J, Enel P, et al. Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS (London, England) 2004;18:2029-38.
37. Lederman MM, McKinnis R, Kelleher D, Cutrell A, Mellors J, Neisler M, et al. Cellular restoration in HIV infected persons treated with abacavir and a protease inhibitor: age inversely predicts naive CD4 cell count increase. AIDS (London, England) 2000;14:2635-42.
38. Le Moing V, Thiebaut R, Chene G, Leport C, Cailleton V, Michelet C, et al. Predictors of long-term increase in CD4(+) cell counts in human immunodeficiency virus-infected patients receiving a protease inhibitor-containing antiretroviral regimen. J Infect Dis 2002;185:471-80.
39. Thiebaut R, Morlat P, Jacqmin-Gadda H, Neau D, Mercie P, Dabis F, et al. Clinical progression of HIV-1 infection according to the viral response during the first year of antiretroviral treatment. Groupe dEpidemiologie du SIDA en Aquitaine (GECSA). AIDS (London, England) 2000;14:971-8.
40. Salzberger B, Rockstroh J, Wieland U, Franzen C, Schwenk A, Jutte A, et al. Clinical efficacy of protease inhibitor based antiretroviral combination therapy-a prospective cohort study. Eur J Med Res 1999;4:449-55.
41. Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA 1999;282:2220-6.
42. Mezzaroma I, Carlesimo M, Pinter E, Muratori DS, Di Sora F, Chiarotti F, et al. Clinical and immunologic response without decrease in virus load in patients with AIDS after 24 months of highly active antiretroviral therapy. Clin Infect Dis 1999;29:1423-30.
43. Kaufmann D, Pantaleo G, Sudre P, Telenti A. CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART). Swiss HIV Cohort Study. Lancet 1998;351:723-4.
44. Ledergerber B, Lundgren JD, Walker AS, Sabin C, Justice A, Reiss P, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet 2004;364:51-62.
45. Phillips A. Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS (London, England) 2004;18:51-8.
46. Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997;126:946-54.
47. Lyles RH, Munoz A, Yamashita TE, Bazmi H, Detels R, Rinaldo CR, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis 2000;181:872-80.
48. Lederman MM, Connick E, Landay A, Kuritzkes DR, Spritzler J, St Clair M, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis 1998;178:70-9.
49. Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 1997;277:112-6.
50. Gray CM, Lawrence J, Schapiro JM, Altman JD, Winters MA, Crompton M, et al. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART). J Immunol 1999;162:1780-8.
51. Ogg GS, Jin X, Bonhoeffer S, Moss P, Nowak MA, Monard S, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol 1999;73:797-800.
52. Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med 1999;5:518-25.
53. Jin X, Ogg G, Bonhoeffer S, Safrit J, Vesanen M, Bauer D, et al. An antigenic threshold for maintaining human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. Mol Med 2000;6:803-9.
54. Garcia F, Plana M, Ortiz GM, Bonhoeffer S, Soriano A, Vidal C, et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS (London, England) 2001;15:F29-40.
55. Ortiz GM, Nixon DF, Trkola A, Binley J, Jin X, Bonhoeffer S, et al. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Invest 1999;104:R13-8.
56. Haslett PA, Nixon DF, Shen Z, Larsson M, Cox WI, Manandhar R, et al. Strong human immunodeficiency virus (HIV)-specific CD4+ T cell responses in a cohort of chronically infected patients are associated with interruptions in anti-HIV chemotherapy. J Infect Dis 2000;181:1264-72.
57. Ruiz L, Ribera E, Bonjoch A, Romeu J, Martínez-Picado J, Paredes R, et al. Role of structured treatment interruption before a 5-drug salvage antiretroviral regimen: the Retrogene Study. J Infect Dis 2003;188:977-85.
58. Lori F, Lewis MG, Xu J, Varga G, Zinn DE Jr, Crabbs C, et al. Control of SIV rebound through structured treatment interruptions during early infection. Science 2000;290:1591-3.
59. Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts < 200 cells/microl. AIDS (London, England)2008;22:237-47.
60. Fagard C, Bandelier CY, Ananworanich J, Le Braz M, Gunthard H, Perneger T, et al. Biphasic decline of CD4 cell count during scheduled treatment interruptions. AIDS (London, England) 2005;19:439-41.
61. Oxenius A, Price DA, Gunthard HF, Dawson SJ, Fagard C, Perrin L, et al. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci USA 2002;99:13747-52.
62. Ruiz L, Carcelain G, Martinez-Picado J, Frost S, Marfil S, Paredes R, et al. HIV dynamics and T-cell immunity after three structured treatment interruptions in chronic HIV-1 infection. AIDS (London, England) 2001;15:F19-27.
63. Carcelain G, Tubiana R, Samri A, Calvez V, Delaugerre C, Agut H, et al. Transient mobilization of human immunodeficiency virus (HIV)-specific CD4 T-helper cells fails to control virus rebounds during intermittent antiretroviral therapy in chronic HIV type 1 infection. J Virol 2001;75:234-41.
64. Davey RT Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA 1999;96:15109-14.
65. Mollet L, Li TS, Samri A, Tournay C, Tubiana R, Calvez V, et al. Dynamics of HIV specific CD8+ T lymphocytes with changes in viral load. The RESTIM and COMET Study Groups. J Immunol 2000;165:1692-704.
66. Hughes MD, Johnson VA, Hirsch MS, Bremer JW, Elbeik T, Erice A, et al. Monitoring plasma HIV-1 RNA levels in addition to CD4+ lymphocyte count improves assessment of antiretroviral therapeutic response. ACTG 241 Protocol Virology Substudy Team. Ann Intern Med 1997;126:929-38.
67. Ghani AC, de Wolf F, Ferguson NM, Donnelly CA, Coutinho R, Miedema F, et al. Surrogate markers for disease progression in treated HIV infection. J Acquir Immune Defic Syndr 2001;28:226-31.
68. Maggiolo F, Ripamonti D, Gregis G, Quinzan G, Callegaro A, Suter F. Effect of prolonged discontinuation of successful antiretroviral therapy on CD4 T cells: a controlled, prospective trial. AIDS (London, England) 2004;18:439-46.
69. Mussini C, Bedini A, Borghi V, Guaraldi G, Esposito R, Barchi E, et al. CD4 cell-monitored treatment interruption in patients with a CD4 cell count > 500 x 106 cells/l. AIDS (London, England) 2005;19:287-94.
70. Fernández Guerrero ML, Rivas P, Molina M, García R, De Gorgolas M. Long-term follow-up of asymptomatic HIV-infected patients who discontinued antiretroviral therapy. Clin Infect Dis 2005;41:390-4.
71. Skiest DJ, Morrow P, Allen B, McKinsey J, Crosby C, Foster B, et al. It is safe to stop antiretroviral therapy in patients with pre-antiretroviral CD4 cell counts >250 cells/microL. J Acquir Immune Defic Syndr 2004;37:1351-7.
72. Ruiz L, Paredes R, Gómez G, Romeu J, Domingo P, Pérez-Alvarez N, et al. Antiretroviral therapy interruption guided by CD4 cell counts and plasma HIV-1 RNA levels in chronically HIV-1-infected patients. AIDS (London, England) 2007;21:169-78.
73. Ananworanich J, Gayet-Ageron A, Le Braz M, Prasithsirikul W, Chetchotisakd P, Kiertiburanakul S, et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet 2006;368:459-65.
74. Skiest D, Havlir D, Coombs R, Adams E, Cain P, Petersen T, et al. Predictors of HIV Disease Progression in Patients Who Stop ART with CD4 Cell Counts >350 cells/mm3 (Session 23 Oral Abstract # 101). 13th Conference on Retroviruses and Opportunistic Infections. Colorado Convention Center, Denver, Colorado,2006.
75. Maggiolo F, Airoldi M, Callegaro A, Martinelli C, Dolara A, Bini T, et al. CD4 cell-guided scheduled treatment interruptions in HIV-infected patients with sustained immunologic response to HAART. AIDS (London, England) 2009;23:799-807.
76. Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, Kanga C, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet 2006;367:1981-9.
77. El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283-96.
78. Ananworanich J, Hirschel B. Intermittent therapy for the treatment of chronic HIV infection. AIDS (London, England) 2007;21:123-34.
79. Camacho R. What have we learned from recent CD4-guided treatment interruption studies. AIDS Rev 2006;8:171-5.
80. Hirschel B, Flanigan T. Is it smart to continue to study treatment interruptions? AIDS (London, England) 2009;23:757-9.
81. García F, Plana M, Vidal C, Cruceta A, OBrien WA, Pantaleo G, et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS (London, England) 1999;13:F79-86.
82. Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS (London, England) 1999;13:F59-62.
83. Hatano H, Vogel S, Yoder C, Metcalf JA, Dewar R, Davey RT Jr, et al. Pre-HAART HIV burden approximates post-HAART viral levels following interruption of therapy in patients with sustained viral suppression. AIDS (London, England) 2000;14:1357-63.
84. Colven R, Harrington RD, Spach DH, Cohen CJ, Hooton TM. Retroviral rebound syndrome after cessation of suppressive antiretroviral therapy in three patients with chronic HIV infection. Ann Intern Med 2000;133:430-4.
85. Kilby JM, Goepfert PA, Miller AP, Gnann JW Jr, Sillers M, Saag MS, et al. Recurrence of the acute HIV syndrome after interruption of antiretroviral therapy in a patient with chronic HIV infection: A case report. Ann Intern Med 2000;133:435-8.
86. Zeller V, Charlois C, Duvivier C, Bricaire F, Katlama C. Pseudo-primary infection syndrome following discontinuation of antiretroviral therapy. Antivir Ther 2001;6:191-3.
87. Ananworanich J, Phanuphak N, Nuesch R, Apateerapong W, Rojnuckarin P, Ubolyam S, et al. Recurring thrombocytopenia associated with structured treatment interruption in patients with human immunodeficiency virus infection. Clin Infect Dis 2003;37:723-5.
88. Neumann AU, Tubiana R, Calvez V, Robert C, Li TS, Agut H, et al. HIV-1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. Comet Study Group. AIDS (London, England) 1999;13:677-83.
89. Cozzi Lepri A, Phillips AN, dArminio Monforte A, Castelli F, Antinori A, deLuca A, et al. When to start highly active antiretroviral therapy in chronically HIV-infected patients: evidence from the ICONA study. AIDS (London, England) 2001;15:983-90.
90. Le Moing V, Chene G, Carrieri MP, Alioum A, Brun-Vezinet F, Piroth L, et al. Predictors of virological rebound in HIV-1-infected patients initiating a protease inhibitor-containing regimen. AIDS (London, England) 2002;16:21-9.
91. Holmberg SD, Hamburger ME, Moorman AC, Wood KC, Palella FJ Jr. Factors associated with maintenance of long-term plasma human immunodeficiency virus RNA suppression. Clin Infect Dis 2003;37:702-7.
92. Phillips AN, Staszewski S, Weber R, Kirk O, Francioli P, Miller V, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA 2001;286:2560-7.
93. Taffe P, Rickenbach M, Hirschel B, Opravil M, Furrer H, Janin P, et al. Impact of occasional short interruptions of HAART on the progression of HIV infection: results from a cohort study. AIDS (London, England) 2002;16:747-55.
94. Wolf E, Hoffmann C, Procaccianti M, Mosthaf F, Gersbacher E, Ulmer A, et al. Long-term consequences of treatment interruptions in chronically HIV-1-infected patients. Eur J Med Res 2005;10:56-62.
95. Salk J. Prospects for the control of AIDS by immunizing seropositive individuals. Nature 1987;327:473-6.
96. Levine AM, Groshen S, Allen J, Munson KM, Carlo DJ, Daigle AE, et al. Initial studies on active immunization of HIV-infected subjects using a gp120-depleted HIV-1 Immunogen: long-term follow-up. J Acquir Immune Defic Syndr Hum Retrovirol 1996;11:351-64.
97. Egan MA. Current prospects for the development of a therapeutic vaccine for the treatment of HIV type 1 infection. AIDS Res Hum Retrovir 2004;20:794-806.
98. Valentine FT, DeGruttola VMK. Effects of HAART compared to HAART plus an inactivated HIV immunogen on lymphocyte proliferative responses (LPR) to HIV antigens. Conference Supplement 12th World AIDS Conference;1998 June 28-July 3; Geneva, Switzerland;1998. p. abstr B31227.
99. Autran B, Costagliola D, Murphy R, Katlama C. Evaluating therapeutic vaccines in patients infected with HIV. Expert Rev Vaccines 2004;3:S169-77.
100. Tryniszewska E, Nacsa J, Lewis MG, Silvera P, Montefiori D, Venzon D, et al. Vaccination of macaques with long-standing SIVmac251 infection lowers the viral set point after cessation of antiretroviral therapy. J Immunol 2002;169:5347-57.
101. Hel Z, Venzon D, Poudyal M, Tsai WP, Giuliani L, Woodward R, et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med 2000;6:1140-6.
102. Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, et al. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol 1999;73:6721-8.
103. Hoff R, McNamara J. Therapeutic vaccines for preventing AIDS: their use with HAART. Lancet 1999;353:1723-4.
104. Autran B, Carcelain G. AIDS. Boosting immunity to HIV- can the virus help?. Science 2000;290:946-9.
105. Hoffmann D, Seebach J, Cosma A, Goebel FD, Strimmer K, Schtzl HM, et al. Therapeutic vaccination reduces HIV sequence variability. FASEB J 2008;22:437-44.
106. Yang H, Dong T, Turnbull E, Ranasinghe S, Ondondo B, Goonetilleke N, et al. Broad TCR usage in functional HIV-1-specific CD8+ T cell expansions driven by vaccination during highly active antiretroviral therapy. J Immunol 2007;179:597-606.
107. Valor L, Navarro J, Carbone J, Rodríguez-Sinz C, Gil J, López F, et al. Immunization with an HIV-1 immunogen induces CD4+ and CD8+ HIV-1-specific polyfunctional responses in patients with chronic HIV-1 infection receiving antiretroviral therapy. Vaccine 2008;26:2738-45.
108. Levy Y, Gahery-Segard H, Durier C, Lascaux AS, Goujard C, Meiffredy V, et al. Immunological and virological efficacy of a therapeutic immunization combined with interleukin- 2 in chronically HIV-1 infected patients. AIDS (London, England) 2005;19:279-86.
109. Robbins GK, Addo MM, Troung H, Rathod A, Habeeb K, Davis B, et al. Augmentation of HIV-1-specific T helper cell responses in chronic HIV-1 infection by therapeutic immunization. AIDS (London, England) 2003;17:1121-6.
110. Gahery H, Daniel N, Charmeteau B, Ourth L, Jackson A, Andrieu M, et al. New CD4+ and CD8+ T cell responses induced in chronically HIV type-1-infected patients after immunizations with an HIV type 1 lipopeptide vaccine. AIDS Res Hum Retrovir 2006;22:684-94.
111. Hejdeman B, Bostrom AC, Matsuda R, Calarota S, Lenkei R, Fredriksson EL, et al. DNA immunization with HIV early genes in HIV type 1-infected patients on highly active antiretroviral therapy. AIDS Res Hum Retrovir 2004;20:860-70.
112. Sandstrom E, Wahren B. Therapeutic immunisation with recombinant gp160 in HIV-1 infection: a randomised double-blind placebo-controlled trial. Nordic VAC-04 Study Group. Lancet 1999;353:1735-42.
113. Kundu SK, Katzenstein D, Moses LE, Merigan TC. Enhancement of human immunodeficiency virus (HIV)-specific CD4+ and CD8+ cytotoxic T-lymphocyte activities in HIV-infected asymptomatic patients given recombinant gp160 vaccine. Proc Natl Acad Sci USA 1992;89:11204-8.
114. Fernández-Cruz E, Moreno S, Navarro J, Clotet B, Bouza E, Carbone J, et al. Therapeutic immunization with an inactivated HIV-1 Immunogen plus antiretrovirals versus antiretroviral therapy alone in asymptomatic HIV-infected subjects. Vaccine 2004;22:2966-73.
115. Kran AM, Sommerfelt MA, Sorensen B, Nyhus J, Baksaas I, Bruun JN, et al. Reduced viral burden amongst high responder patients following HIV-1 p24 peptide-based therapeutic immunization. Vaccine 2005;23:4011-5.
116. MacGregor RR, Boyer JD, Ugen KE, Tebas P, Higgins TJ, Baine Y, et al. Plasmid vaccination of stable HIV-positive subjects on antiviral treatment results in enhanced CD8 T-cell immunity and increased control of viral blips. Vaccine 2005;23:2066-73.
117. Tubiana R, Carcelain G, Vray M, Gourlain K, Dalban C, Chermak A, et al. Therapeutic immunization with a human immunodeficiency virus (HIV) type 1-recombinant canarypox vaccine in chronically HIV-infected patients: The Vacciter Study (ANRS 094). Vaccine 2005;23:4292-301.
118. OBrien WA, Grovit-Ferbas K, Namazi A, Ovcak-Derzic S, Wang HJ, Park J, et al. Human immunodeficiency virus-type 1 replication can be increased in peripheral blood of seropositive patients after influenza vaccination. Blood 1995;86:1082-9.
119. Farber CM, Barath AA, Dieye T. The effects of immunization in human immunodeficiency virus type 1 infection. N Engl J Med 1996;335:817;author reply 8-9.
120. Kroon FP, Van Furth R, Bruisten SM. The effects of immunization in human immunodeficiency virus type 1 infection. N Engl J Med 1996;335:817-8;author reply 8-9.
121. Stanley SK, Ostrowski MA, Justement JS, Gantt K, Hedayati S, Mannix M, et al. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med 1996;334:1222-30.
122. García JA, Wu FK, Mitsuyasu R, Gaynor RB. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J 1987;6:3761-70.
123. Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 1987;326:711-3.
124. Yerly S, Wunderli W, Wyler CA, Kaiser L, Hirschel B, Suter S, et al. Influenza immunization of HIV-1-infected patients does not increase HIV-1 viral load. AIDS (London, England) 1994;8:1503-4.
125. Glesby MJ, Hoover DR, Farzadegan H, Margolick JB, Saah AJ. The effect of influenza vaccination on human immunodeficiency virus type 1 load: a randomized, doubleblind, placebo-controlled study. J Infect Dis 1996;174:1332-6.
126. Jackson CR, Vavro CL, Valentine ME, Pennington KN, Lanier ER, Katz SL, et al. Effect of influenza immunization on immunologic and virologic characteristics of pediatric patients infected with human immunodeficiency virus. Pediatr Infect Dis J 1997;16:200-4.
127. Kolber MA, Gabr AH, De La Rosa A, Glock JA, Jayaweera D, Miller N, et al. Genotypic analysis of plasma HIV-1 RNA after influenza vaccination of patients with previously undetectable viral loads. AIDS (London, England) 2002;6:537-42.
128. Tasker SA, OBrien WA, Treanor JJ, Weiss PJ, Olson PE, Kaplan AH, et al. Effects of influenza vaccination in HIV-infected adults: a double-blind, placebo-controlled trial. Vaccine 1998;16:1039-42.
129. Gunthard HF, Wong JK, Spina CA, Ignacio C, Kwok S, Christopherson C, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis 2000;181:522-31.
130. Fuller JD, Craven DE, Steger KA, Cox N, Heeren TC, Chernoff D. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis 1999;28:541-7.
131. Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian R, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS (London, England) 2003;17:2015-23.
132. Emery S, Workman C, Puls RL, Bloch M, Baker D, Bodsworth N, et al. Randomized, placebo-controlled, phase I/IIa evaluation of the safety and immunogenicity of fowlpox virus expressing HIV gag-pol and interferongamma in HIV-1 infected subjects. Hum Vaccin 2005;1:232-8.
133. Murray JS, Elashoff MR, Iacono-Connors LC, Cvetkovich TA, Struble KA. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS (London, England) 1999;13:797-804.
134. Follmann D, Duerr A, Tabet S, Gilbert P, Moodie Z, Fast P, et al. Endpoints and regulatory issues in HIV vaccine clinical trials: lessons from a workshop. J Acquir Immune Defic Syndr 2007;44:49-60.
135. Smith KA. To cure chronic HIV infection, a new therapeutic strategy is needed. Curr Opin Immunol 2001;13:617-24.
136. Verrier B. Therapeutic vaccination for chronic infectious diseases: lessons from HIV-1. J Clin Virol 2005;34 Suppl 1:S9-S12.
137. Kinloch-de Loes S, Hoen B, Smith DE, Autran B, Lampe FC, Phillips AN, et al. Impact of therapeutic immunization on HIV-1 viremia after discontinuation of antiretroviral therapy initiated during acute infection. J Infect Dis 2005;192:607-17.
138. Walensky RP, Paltiel AD, Goldie SJ, Gandhi RT, Weinstein MC, Seage GR 3rd, et al. A therapeutic HIV vaccine: how good is good enough? Vaccine 2004;22:4044-53.
139. Executive summary and recommendations from the WHO/UNAIDS/IAVI expert group consultation on Phase IIB-TOC trials as a novel strategy for evaluation of preventive HIV vaccines , 31 January-2 February 2006, IAVI, New York, USA. AIDS (London, England)2007; 1:539-46.
140. Excler JL, Rida W, Priddy F, Fast P, Koff W. A strategy for accelerating the development of preventive AIDS vaccines. AIDS (London, England) 2007;21:2259-63.
141. Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol 2001;2:349-51.
142. Goetz MB, Boscardin WJ, Wiley D, Alkasspooles S. Decreased recovery of CD4 lymphocytes in older HIV-infected patients beginning highly active antiretroviral therapy. AIDS (London, England) 2001;15:1576-9.
143. Teixeira L, Valdez H, McCune JM, Koup RA, Badley AD, Hellerstein MK, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS (London, England) 2001;15:1749-56.
144. Marimoutou C, Chne G, Merci P, Neau D, Farbos S, Morlat P, et al. Prognostic factors of combined viral load and CD4+ cell count responses under triple antiretroviral therapy, Aquitaine cohort, 1996-1998. J Acquir Immune Defic Syndr 2001;27:161-7.
145. Fernández S, Nolan RC, Price P, Krueger R, Wood C, Cameron D, et al. Thymic function in severely immunodeficient HIV type 1-infected patients receiving stable and effective antiretroviral therapy. AIDS Res Hum Retrovir 2006;22:163-70.
146. Robbins GK, Spritzler JG, Chan ES, Asmuth DM, Gandhi RT, Rodriguez BA, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis 2009;48:350-61.
147. Kolber MA, Saenz MO, Gmez-Marn O, Tamariz LJ. Race and ethnicity impact on the maximum proliferative response in peripheral blood lymphocytes from HIV-seropositive individuals. HIV Med 2007;8:401-5.
148. Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 2004;44:499-523.
Received in January, 2009.
Accepted for publication in September, 2009.
Enrique Iglesias. Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 entre 158 y 190, Cubanacán, Playa, CP 10 400, AP 6162, Ciudad de La Habana, Cuba. E-mail: enrique.iglesias@cigb.edu.cu