Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.26 n.3 La Habana jul.-sep. 2009
REPORT
Tilapia somatotropin polypeptides: potent enhancers of fish growth and innate immunity *
Jannel Acosta1, Mario P Estrada1, Yamila Carpio1, Odalys Ruiz2, Reynold Morales1, Eduardo Martínez2, Jorge Valdés2, Carlos Borroto1, Vladimir Besada3, Aniel Sánchez3, Fidel Herrera1
1Aquatic Biotechnology Project. Division of Animal Biotechnology
2Development Division, Center for Genetic Engineering and Biotechnology, Havana, Cuba
3Chemical-Physical Division
Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 / 158 and 190, Playa, PO Box 6162, Havana, Cuba
ABSTRACT
Growth hormone (GH) is a single chain polypeptide of approximately 22 kDa, produced by the pituitary gland and with pleiotropic functions among vertebrates. It mainly regulates body growth, being also involved in reproduction, immunity and osmoregulation in teleost fish. In order to obtain the tilapia (Oreochromis hornorum) growth hormone (tiGH) in Pichia pastoris cells, its gene was cloned into expression vectors in both with and without a heterologous secretion signal. The tiGH, obtained either intracellularly or extracellularly in P. pastoris cells, was characterized showing its production as associated to the cellular rupture precipitate with an approximate molecular weight of 22 kDa; or being secreted with an approximate molecular weight of 18 kDa, respectively. The mass spectrometry analysis of the recombinant protein obtained in the culture supernatant corroborated the identity of the protein as tiGH but lacking 46 aminoacids of its carboxyl terminal sequence. The tiGH biological activity of P. pastoris intact cells producing this protein was carried out in tilapia larvae (Oreochromis sp.), showing, for the first time, that it is possible to stimulate fish growth by immersion baths with recombinant tiGH-producing yeast. On the contrary what was previously postulated for mammals, the evaluation of P. pastoris cells expressing the truncated variant of tiGH demonstrated that this protein is also able to stimulate growth and immune system in fish. This is the first report of a biologically active, truncated GH variant in fish.
INTRODUCTION
The development of Aquatic Biotechnology has supported the application of experimental techniques to manipulate fish growth, as diets enriched in specific proteic nutrients and administrating hormones like: prolactin, insulin and growth hormone (GH) (1).
GH is a single chain polypeptide of approximately 22 kDa, produced by the pituitary gland and with pleiotropic functions among vertebrates. It mainly regulates body growth, being also involved in reproduction, immunity and osmosis regulation in teleost fish, and in metabolism regulation through its lipolytic activity and protein anabolism in vertebrates (2).
The structure of fish GH molecule remains unknown. Otherwise, it has been suggested based on aminoacid sequence alignments that it bears functional epitopes similar to the human GH (3) which comprises four anti-parallel ahelixes separated by connecting loops as well as four Cys residues located at positions 53, 165, 182 and 189 loops and with four cystein residues (4). These residues are highly conserved between GH molecules from fish to mammals (5, 6), suggesting that they may be involved in structural integrity and/or biological activity of the hormone (7). The information about the interaction of GH with its receptor(s) in fish is scarce, and there are discrepancies on receptors identity. Only one study has proposed that a one-hormone-two-receptors model applies in fish and possibly to all the vertebrates (8). That study has also postulated the presence of receptor binding site 1 in the goldfish GH-II molecule, comprising three discontinuous regions located in helix 1, together with residues of the middle portion of the molecule and helix 4, and that receptor binding site 2 is located in the middle part of the molecule (8).
GH has also been recognized as relevant for the aquatic industry due to its role on growth and as immune stimulator (9-12). Hence, the genes coding for GH in different fish species have been cloned and expressed in yeasts and Escherichia coli cells (13-15).
Several studies have been conducted to show the growth-stimulating effect of recombinant GH administered by several routes including injection (14-16), oral incubation (17), immersion baths (18-20) and dietary delivery (21-23). Among these methods, oral incubation and immersion bath are the most feasible for aquaculture avoiding individual manipulation of fish (10, 20, 24). There are several works demonstrating growth stimulation by feeding fish with diets enriched of GH-producing yeasts (25-27). However, none of them evaluated the possible effects of GH by supplying GH-producing cells without any other additive and by using immersion procedures. The previous purification GH is the main factor making its use more expensive and prohibitive to be used in aquaculture. The aim of this work was to evaluate for the first time the effect of administering by immersion baths tilapia GH (tiGH)-producing yeast cells or their culture supernatants containing secreted tiGH, to stimulate growth and immune functions in larval and juvenile fish.
RESULTS AND DISCUSSION
Obtaining the tilapia growth hormone intracellularly in Pichia pastoris cells
The DNA fragment coding for tiGH was PCR-amplified from the pTTI plasmid, lacking its signal peptide for intracellular expression (14), and was inserted into the pNAO vector to obtain the pP-tiGH plasmid with the aim of obtaining a P. pastoris strain producing tiGH intracellularly. It bears an integration unit composed of homologous regions to the AOX1 locus of P. pastoris genome (5AOX1 and 3AOX1) for its integration by homologous recombination. The pP-tiGH was digested with the Cla I endonuclease and used for transforming of P. pastoris cells strain MP36 by electroporation. Southern-blot analyses of three genomic DNA, isolated from three transformants showed a 2.8 kb band in two of them, indicative of double crossovers between the expression cassette and the endogenous AOX1 gene in the host genomes (gene replacement integration)(See Figure 2 in reference (28)). This event generates clones of slow methanol consumption (MutS) (29). The genomic DNA of the untransformed P. pastoris strain MP36 was used as negative control of the assay, being detected the 5.5 kb-band corresponding to the native AOX1 gene. Trans-formant C9 was denominated MP36/pPtiGH and selected for further expression studies.
The expression of tiGH from the MP36/pP-tiGH strain was analyzed in the intracellular fraction under reducing conditions by 15% SDS-PAGE, showing a protein band of an approximate molecular weight between 21 and 31 kDa (Figure 3 in reference (28)). Its molecular weight was similar to the 22 kDa of the tiGH molecule previously obtained in E. coli cells. Protein identity was corroborated by Western blot analyses with the aid of an anti-tiGH-HRP monoclonal antibody (Figure 2B in reference (28)). This recombinant protein was absent in analyzed samples of the native MP36 strain.
The intracellular tiGH obtained in P. pastoris cells reached approximately 4.3% of the total protein content. This value was determined by optical densitometry from 15% SDS-PAGE gels (Figure 2A in reference (28)). Moreover, the cellular density at the end of culture was approximately of 300 g/L as determined from the wet biomass weight. Recombinant tiGH expression levels were estimated by protein Dot blot, with the aid of a tiGH standard obtained in E. coli of known concentration (14), reaching approximately 1.5 g/L of culture (data not shown).
Effect of tiGH from the MP36/pP-tiGH strain on growth of red tilapia (Oreochromis sp.) larvae
An experiment was carried out to evaluate the biological activity of tiGH produced by the MP36/pP-tiGH P. pastoris strain on stimulating growth of 3-days posthatching tilapia larvae. Intact yeast cells containing tiGH were administered by immersion baths, thrice a week for 6 weeks. Larvae body weight increased significantly four weeks after starting treatment with P. pastoris tiGH-producing cells, compared to the negative control group treated with untransformed P. pastoris cells (p < 0.0001) (Figure 4 in reference (28)). Although the stimulatory effect of recombinant GH being administered by immersion baths has been previously demonstrated in fish (18, 20), this is the first report using P. pastoris cells expressing a fish GH for that purpose. Previous demonstrations have only used diets enriched with GH-expressing yeast (25-27).
Obtaining an extracellular variant of tiGH in P. pastoris cells
The PCR-amplified 0.8 kb DNA fragment from the pTTI plasmid (14) that codes for tiGH was cloned into the pPS7 vector in frame between the Saccharomyces cerevisiae suc signal peptide sequence and the GAP terminator, generating the construct pPS7-tiGH. This vector bears an AOX1 homologous integration unit similar to that described above for the pP-tiGH construct.
The pPS7-tiGH construct was digested with the Sph I restriction endonuclease and used for transforming of P. pastoris cells strain MP36 by electroporation. Southern blot analysis of genomic DNA showed four out of five transformants with a DNA band of 28.7 kb indicating the homologous integration by double crossover of the expression cassette (integration by AOX1 gene replacement) (Figure 1 in reference (30)). These clones showed the slow methanol consumption (MutS) phenotype (29). The 5.5 kb band corresponding to the native host AOX1 gene was observed in the genomic DNA of the control, untransformed P. pastoris MP36 strain. The transformant corresponding to lane 1 in Figure 1 (30) was selected for expression studies, being denominated MP36/ pPS7-tiGH.
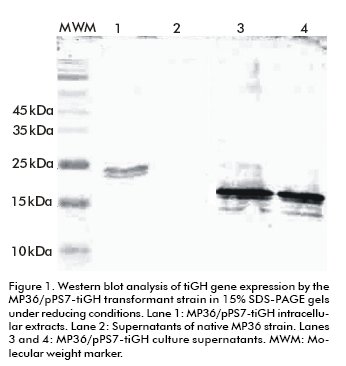
The Western blot analysis of culture supernatants from 15% SDS-PAGE under reducing conditions showed a protein band immunoreactive to an anti-tiGH monoclonal antibody. Its molecular weight was between 15 and 25 kDa (Figure 1), lower than both the 22 kDa native tiGH (14) and the intracellular tiGH produced in P. pastoris cells (28). The recombinant protein was absent in culture supernatants of the native untransformed MP36 strain. Its expression levels were estimated by Dot blot, comparing the previously mentioned tiGH standard obtained in E. coli (14), reaching 40 and 80 mg/L (data not shown). Mass spectrometry analyses corroborated the identity of the protein as tiGH but lacking the 46 carboxyl A similar experiment to evaluate the intracellular tiGH effect on growth of tilapia larvae was carried out, but using this time culture supernatants from the MP36/pPS7-tiGH strain containing the truncated tigGH to stimulate growth of 3-days post-hatching tilapia larvae. Treatment was applied by immersion baths thrice a week for 7 weeks. As for the intracellular variant, larvae body weight increased significantly four weeks after starting treatment with culture supernatants from MP36/pPS7-tiGH cells, compared to the negative control group treated with supernatants from untransformed P. pastoris cells (p < 0.0001) (Figure 2), reaching a 2.2-fold increase after 7 weeks (p < 0.0001) (Figure 2). These results demonstrated that the truncated tiGH variant lacking the 46 carboxyl terminal aminoacids from the native tiGH is biologically active.
Comparison of biological activities of the cellular lysate and culture supernatant from the respective intracellular and truncated tiGH variants-expressing cells
The stimulatory biological effects of both tiGH variants (intracellular complete tiGH or secreted truncated tiGH) on the innate immunity and growth of tilapia larvae were evaluated in of 3-days post-hatching larvae. Treatment was applied by immersion baths thrice a week for 45 days. Larvae were analyzed at 21 and 45 days after the start of the experiment for growing parameters and innate immune functions (lysozyme and hemmaglutination activities).
Growth stimulating activity
A significant increase in body weight was observed in day 21 for tilapia larvae treated with culture supernatants containing the truncated tiGH, compared to both the larvae control group (p < 0.001) and the group treated with lysates of the untransformed native P. pastoris strain (p < 0.05) (Figure 4 in reference (30)). Moreover, larvae treated with intracellular tiGH-containing P. pastoris cell lysates also significantly increased body weight compared to the untreated control group (p < 0.001) (Figure 4 in reference (30)).
Larvae treated with supernatants containing the truncated tiGH showed the best growing results 45 days after treatment. They had a 3.6-fold increase of body weight compared to the untreated group (p < 0.001), a 2.1-fold increase treated with untransformed P. pastoris culture supernatants (p < 0.001), 2.9- fold in the group treated with untransformed P. pastoris cell lysates (p < 0.001) and 1.6-fold in the group treated with intracellular tiGH-containing cell lysates (p < 0.05) (Figure 4 in reference (30)). Likewise, the group treated with the intracellular tiGH-containing lysates showed a 2.2- and 1.8-fold increase in body weight compared to the untreated control group (p < 0.001) and the group treated with untransformed P. pastoris cell lysates (p < 0.001), respectively (Figure 4 in reference (30)).
Effects of tiGH variants on innate immunity
Lysozyme activity
Lysozyme activity was undetectable in larvae homogenates 21 days after starting treatment. There were statistically significant differences at day 45 only between the group treated with culture supernatants containing truncated tiGH compared to the untreated control group (p < 0.05) (Table 1 in reference (30)).
Hemagglutinating activity
There were no statistically significant differences 21 days after beginning treatment. Consistently, after 45 days, the highest hemagglutinating activity was obtained in the group treated with truncated tiGH-containing supernatants (1024 ± 1), statistically significant when compared to the untreated control group (128 ± 1; p < 0.001) and groups treated either with untransformed P. pastoris culture supernatants (512 ± 1; p < 0.01), untransformed P. pastoris cell lysates (256 ± 1; p < 0.001) or intracellular tiGH-containing cell lysates (323 ± 2.5; p < 0.001) (Figure 5 in reference (30)). All the treatments stimulate haemagglutinin activity compared to non-treated group (Figure 5 in reference (30)). No differences among groups were found at day 21 (data not shown). This was the first report linking GH administration to lectin activation. In this sense, we cannot exclude a synergic or individual influence on this parameter of some factors present in culture supernatants activating innate immunity in fish, coming either from P. pastoris cells (intracellular and/ or secreted) or even from culture medium components. As far as we know, there are no reports on this matter. Nevertheless, it has been reported that S. cerevisiae cell wall components such as β-1,3-D-glucan, B-1,6- D-glucan, mannoproteins and chitin (31) individually increase innate defense mechanisms and/or disease resistance when purified ad administered to vertebrates (32-34). B-glucans and mannoproteins are the main yeast cell wall components. The former enhance fish humoral (lysozyme and complement activity) and cellular (phagocytic and respiratory burst activities and bacterial killing) immune responses both in vitro and in vivo (35, 36). The least abundant yeast cell wall compound, but one that is very important, is chitin. Some studies have shown that chitin and its derivatives modulate the immune system in mammals as well as in fish (33, 34, 37). Finally, the combination of all the yeast cell compounds, not only cell wall sugars but also vitamins and genetic material, might produce an optimum physiological status in fish due to multiple interactions, particularly with regard to the immune system (38-40). In addition, the effect of GH by itself can be either direct by interacting with hormone regulators of the immune response or indirect by just accelerating the development of larval immune cell populations. All these require further investigation.
This is the first report of a tiGH polypeptide fragment lacking the last 46 aminoacids of the native tiGH which shows in vivo biological activity for stimulating growth and being involved in innate immune stimulation of tilapia larvae. Differences observed between the truncated and the intracellular tiGH variants cannot be attributed to the tiGH molecule itself, since both variants were tested coming from complex samples subjected to different procedures and none of them included tiGH purification. Moreover, they were supplied at different tiGH molar concentrations and without knowing the possible influence of their different tiGH molecular tertiary structures.
Based on these facts, we decided to use the culture supernatant containing the truncated tiGH molecule for further studies of its industrial application in aquaculture.
Effect of truncated tiGH-containing supernatant of P. pastoris cells in ornamental fish
Effect on growth and survival of goldfish larvae (Carassius auratus)
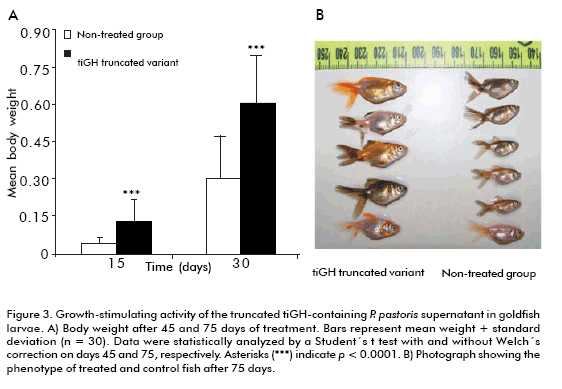
The effect of truncated tiGH-containing supernatant on growth and survival of goldfish larvae was evaluated from 5-days post-hatching larvae. Treatment was applied by immersion baths thrice a week for 75 days. Treated larvae showed a 3.1-fold increase in body weight compared to the untreated group (p < 0.0001; Figure 3) after 45 days of started treatment, that remained two-fold after 75 days (p < 0.0001; Figure 3), also showing increased coloration (Figure 3B). Besides, truncated tiGH-containing supernatant also enhanced survival 22% after 75 days, compared to the animals of the control group.
Effect on growth of larvae and juvenile carp (Cyprinus carpio)
The truncated tiGH-containing supernatant was also evaluated in different developmental stages of trout (Cyprinus carpio). For this purpose, 5 days post-hatching larvae and juveniles (1.2 ± 0.2 g) were treated by immersion baths thrice a week. Larvae were sampled from 15 to 30 days after starting the experiment, showing a 3.5-fold body weight increase compared to the control group (p < 0.0001) at day 15, which remained 2.2-fold (p < 0.0001) after 30 days (Figure 4).
Trout juveniles were sampled 0, 15 and 28 days showing significant increased body weight compared to the control group (p < 0.05), which was 1.5-fold after 28 days (p < 0.0001) (Figure 5).
Effect on growth and anatomical parameters of angelfish (Pterophyllum scalare) juveniles
The effect of truncated tiGH-containing supernatant on growth and quality-related parameters of ornamental fish was also evaluated in angelfish juveniles (3.1 ± 0.6 g) receiving immersion baths thrice a week. Fish showed gained weight 1.6-fold after 30 days than those of the untreated group (p < 0.01, Figure 6). Besides, their body length (p < 0.01), width (p < 0.01) and length of the dorsal fin (p < 0.001) significantly increased in the treated group (Table 1). These results demonstrate that treatment with truncated tiGH-containing supernatants not only increases growth, but also stimulates quality-related body parameters in ornamental fish. It was also demonstrated its action in larvae and juvenile fish developmental stages.
CONCLUSIONS
For the first time, these findings provide differences of the hormone receptor interaction and the structurefunction relationship between fish and mammals, as well contribute to understand the interactions of GH with its receptors in fish. Moreover, because the truncated tiGH variant being supplied in P. pastoris culture supernatants showed to be a potent enhancer for growth, survival, quality of larvae and immune parameters, it can be used in aquaculture mainly for ornamental fish production. It allows to have treated fish of increased growing rates and more resistant to stress conditions and infections, and reducing production costs.
REFERENCES
1. Pullin RS, McConell RH. The biology and culture of tilapias. Conference Proceeding, 1982; International Center for living Aquatic Resources Management.
2. Rousseau K, Dufour S. Comparative aspects of GH and metabolic regulation in lower vertebrates. Neuroendocrinology 2007;86:165-74.
3. Chen TT, Marsh A, Shamblott M, Chan KM, Tang YL, Cheng CM, et al. Structure and evolution of fish growth hormone and insulin-like growth factors genes. In: Sherwood NM, and Hew CL (Eds.), Fish Physiology. Molecular Endocrinology of fish, Academic Press, San Diego,1994; pp. 79-209.
4. de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 1992;255:306-12.
5. Nicoll CS, Mayer GL, Russell SM. Structural features of prolactins and growth hormones that can be related to their biological properties. Endocr Rev 1986;7:169-203.
6. Watahiki M, Yamamoto M, Yamakawa M, Tanaka M, Nakashima K. Conserved and unique amino acid residues in the domains of the growth hormones. Flounder growth hormone deduced from the cDNA sequence has the minimal size in the growth hormone prolactin gene family. J Biol Chem 1989;264:312-6.
7. Kopchick JJ. Growth and maturation. In: Melmed S (Ed.), Endocrinology. WB Saunders Co., Philadelphia, 2001; pp. 89-404.
8. Chan YH, Cheng CH, Chan KM. Study of goldfish (Carassius auratus) growth hormone structure-function relationship by domain swapping. Comp Biochem Physiol B Biochem Mol Biol 2007;146:384-94.
9. Sakai M, Kajita Y, Kobayashi M, Kawauchi H. Immunostimulating effect of growth hormone: in vivo administration of growth hormone in rainbow trout enhances resistance to vibrio anguillarum infection. Vet Immunol Immunopathol 1997;57:147-52.
10. Jeh HS, Kim CH, Lee HK, Han K. Recombinant flounder growth hormone from Escherichia coli: overexpression, efficient recovery, and growth-promoting effect on juvenile flounder by oral administration. J Biotechnol 1998;60:183-93.
11. Farmanfarmaian A, Sun LZ. Growth hormone effects on essential amino acid absorption, muscle amino acid profile, N-retention and nutritional requirements of striped bass hybrids. Genet Anal 1999;15:107-13.
12. Yada T, Muto K, Azuma T, Ikuta K. Effects of prolactin and growth hormone on plasma levels of lysozyme and ceruloplasmin in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2004;139:57-63.
13. Tsai HJ, Tseng CF, Kuo TT. Expression of rainbow trout growth hormone cDNA in yeast. Bull Inst Zool Acad Sin 1993;32:162-70.
14. Guillén II, Lleonart R, Agramonte A, Morales R, Hernández CA, Vázquez MA, et al. Physiological changes in the juvenile euryhaline teleost, the tilapia Oreochromis hornorum, injected with E. coli-derived homologous growth hormone. J Mar Biotechnol 1998;6:142-51.
15. Li Y, Bai J, Jian Q, et al. Expression of common carp growth hormone in the yeast Pichia pastoris and growth stimulation of juvenile tilapia (Oreochromis niloticus). Aquaculture 2003;216:329-41.
16. Baile CA, la-Fera MA, McLaughlin CL. Performance and carcass quality of swine injected daily with bacterially-synthesized human growth hormone. Growth 1983;47:225.
17. Hertz Y, Tchelet A, Madar Z, Gertler A. Absorption of bioactive human growth hormone after oral administration in the common carp (Cyprinus carpio) and its enhancement by deoxycholate. J Comp Physiol B 1991;161:159-63.
18. Agellon LB, Emery CJ, Jones JM, Davies SL, Dingle AD, Chen TT. Promotion of rapid growth of rainbow trout (Salmo gairdneri) by a recombinant fish growth hormone. Can J Fish Aquat Sci 1988;45:146-51.
19. Schulte PM, Down N, Donaldson EM, Souza LM. Experimental administration of recombinant bovine growth hormone to juvenile rainbow trout (Salmo gairdneri) by injection or by immersion. Aquaculture(Amsterdam) 1989;76:145-56.
20. Moriyama S, Kawauchi H. Growth stimulation of juvenile salmonids by immersion in recombinant salmon growth hormone. Nippon Suisan Gakkaishi 1990;56:31-4.
21. McLean E, Donaldson EM, Teskeredzic E, Souza LM. Growth enhancement following dietary delivery of recombinant porcine somatotropin to diploid and triploid coho salmon (Oncorhynchus kisutch). Fish Physiol Biochem 1993;11:363-69.
22. Tsai HJ, Hsih MH, Kuo JC. Escherichia coli-produced fish growth hormone as a feed additive to enhance the growth of juvenile black seabream (Acanthopagrus schlegeli). J Appl Ichthyol 1997;13:79-82.
23. Ben-Atia I, Fine M, Tandler A, Funkensteinb B, Mauricec S, Cavarib B, et al. Preparation of recombinant gilthead seabream (Sparus aurata) growth hormone and its use for stimulation of larvae growth by oral administration. Gen Comp Endocrinol 1999;113:155-64.
24. Moriyama S, Yamamoto H, Sugimoto S, Abe T, Hirano T, Kawauchi H. Oral administration of recombinant salmon growth hormone to rainbow trout, Oncorhynchus mykiss. Aquaculture (Amsterdam) 1993;112:99-106.
25. Tsai HJ, Wei KC, Ching CC. Enhancement of tilapia growth by dietary administration of recombinant yeast lysates as a supplement. J Fish Soc Taiwan 1993;20:339-45.
26. Tsai H, Kuo J, Lou S, Kuo T. Growth enhancement of juvenile striped mullet by feeding recombinant yeasts containing fish growth hormone. Prog Fish Cult 1994;56:7-12.
27. Li Y, Bai J, Li X, Ye X, Jian Q, Luo JR, et al. Expression of common carp growth hormone in yeast P. pastoris. Chin J Biochem Mol Biol 2001;17:488-91.
28. Acosta J, Morales R, Morales A, Alonso M, Estrada MP. Pichia pastoris expressing recombinant tilapia growth hormone accelerates the growth of tilapia. Biotechnol Lett 2007;29:1671-6.
29. Cregg JM and Malden KR. Development of yeast transformation system and construction of methanol utilization defective mutants of Pichia pastoris by disruption. In: Stewart GG, Rusel I, Klein RD, RR. (Eds), Biological Research on Industrial yeast. CRC Press, Boca Raton, FL, 1987; pp. 1-18.
30. Acosta J, Carpio Y, Besada V, Morales R, Sánchez A, Curbelo Y, et al. Recombinant truncated tilapia growth hormone enhances growth and innate immunity in tilapia fry (Oreochromis sp.). Gen Comp Endocrinol 2008;157:49-57.
31. Cabib E, Roberts R, Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem 1982;51:763-93.
32. Engstad RE, Robertsen B. Specificity of a beta-glucan receptor on macrophages from Atlantic salmon (Salmo salar L.). Dev Comp Immunol 1994;18:397-408.
33. Esteban MA, Mulero V, Cuesta A, Ortuno J, Meseguer J. Effects of injecting chitin particles on the innate immune response of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 2000;10:543-554.
34. Esteban MA, Cuesta A, Ortuno J, Meseguer J. Immunomodulatory effects of dietary intake of chitin on gilthead seabream (Sparus aurata L.) innate immune system. Fish Shellfish Immunol 2001;11:303-315.
35. Thompson I, Choubert G, Houlihan DF, Secombes CJ. The effect of dietary vitamin A and astaxanthin on the immunocompetence of rainbow trout. Aquaculture 1995;133:91-102.
36. Duncan PL, Klesius PH. Dietary immunostimulants enhance nonspecific immune responses in channel catfish but not resistance to Edwardsiella ictaluri. J Aquat Anim Health 1996;8:241-248.
37. Cuesta A, Esteban M, Meseguer J. In vitro effect of chitin particles on the innate cellular immune system of gilthead sea bream (Sparus aurata L.). Fish Shellfish Immunol 2003;15:1-11.
38. Kulkarni AD, Fanslow WC, Rudolph FB, Van Buren CT. Modulation of delayed hypersensitivity in mice by dietary nucleotide restriction. Transplantation 1987;44:847-849.
39. Rudolph FB, Kulkarni AD, Fanslow WC, Pizzini RP, Kumar S, Van Buren CT. Role of RNA as a dietary source of pyrimidines and purines in immune function. Nutrition 1990;6:45-52.
40. Cerra FB, Lehmann S, Konstantinides N, Dzik J, Fish J, Konstantinides F, et al. Improvement in immune function in ICU patients by enteral nutrition supplemented with arginine, RNA, and menhaden oil is independent of nitrogen balance. Nutrition 1991;7:193-199.
*This work received the Award of the National Academy of Sciences of Cuba for the year 2008.
Jannel Acosta. Aquatic Biotechnology Project. Division of Animal Biotechnology. Center for Genetic Engineering and Biotechnology, CIGB. Ave. 31 / 158 and 190, Playa, PO Box 6162, Havana, Cuba. E-mail: jannel.acosta@cigb.edu.cu