My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.26 no.4 La Habana Oct.-Dec. 2009
RESEARCH
Comparative immunogenicity and protective capacity of two Dengue-4 vaccine candidates based on P64k-envelope domain III
Evaluación inmunológica en ratones de dos candidatos vacunales basados en la proteína P64k y el dominio III de la proteína de la envoltura del virus Dengue-4
Laura Lazo, Lázaro Gil, Aída Zulueta, Iris Valdés, Yordanka Soria, Yaremis Romero,Gerardo Guillén, Lisset Hermida
Center for Genetic Engineering and Biotechnology, CIGB. Ave. 31 / 158 and 190, Playa, PO Box 6162, Havana, Cuba
ABSTRACT
In this work we compared, in the same immunization schedule in mice, two vaccine candidates against Dengue-4 based on the envelope protein domain III and the carrier P64k. Their molecular and antigenic features, immunogenicity and protective capacity in the viral encephalitis murine model are assessed. Our study endorses the strategy of using both sites of inclusion within the same P64k molecule, in order to enhance the immunogenicity of protein fragments.
Keywords: Dengue, vaccine, P64k, carrier
RESUMEN
En este trabajo comparamos en el mismo esquema de inmunización en ratones, dos candidatos vacunales contra el virus Dengue-4 basados en el dominio III de la proteína de la envoltura y la proteína portadora P64k. Además caracterizamos antigénicamente y molecularmente ambas variantes proteicas y evaluamos la inmunogenicidad y capacidad protectora en el modelo murino de encefalitis viral. Este estudio avala la estrategia de emplear a la misma vez, dos sitios de inclusión en la P64k como proteína portadora, con el objetivo de potenciar la inmunogenicidad de fragmentos proteicos heterólogos.
Palabras clave: Dengue, vacuna, P64k, portadora
INTRODUCTION
The envelope protein is the major surface protein of Dengue virus (DEN) and its domain III has been con-sidered to function as the putative cellular receptor-binding domain (1). This viral fragment has been wi-dely evaluated in vaccine candidates against DEN employing the maltose binding protein from Escherichia coli, the protein A from Staphylococcus aureus and the trp-e gene from E. coli, as carriers (2- 4). Unfortunately, the safety of these carrier proteins has not yet been evaluated in humans. On the other hand, in the purification protocols of these fusion constructs, affinity chromatography is required, which increase production costs of the antigens. As an alternative, the P64k protein has also been employed as a carrier for the domain III of DEN envelope protein (5-8).
The P64k protein is a lipoamide dehydrogenase from Neisseria meningitidis serogroup B (9). Its safety in humans has been proved with successful results (10). Due to its immunogenicity, availability and molecular weight, this protein has been widely evaluated as a carrier for weak immunogens such as synthetic peptides and the meningococcal serogroup C polysaccharide (11-12).
Two sites inside the P64k protein for the inclusion of the DEN fragment have been reported: i) after the first 45 amino acids, within the region coding for the lipoil binding domain (LBD) (insertion variant), and ii) the C-terminus (fusion variant) (5- 8). In 2003, the molecular characterization of the two protein variants corresponding to dengue serotype 4 (DEN4) was reported. Both proteins showed similar antigenic featu res by ELISA using polyclonal antibodies; however the insertion variant exhibited a high level of degradation after the semi-purification process (8). On the other hand, experiments conducted in monkeys showed that the fusion variant of P64k-domain III of DEN2 exhibited a higher immunogenicity and protective capacity than its insertion counterpart (7).
Based on these results, we selected the recombinant protein resulting from the fusion of DEN4 envelope domain III to the C-terminus of P64k (hereinafter PD19) to perform the immunological evaluations in animals (unpublished data). In agreement with previous findings for other DEN4 vaccine candidates (2, 13, 14), PD19 resulted poorly immunogenic compared with candidates for the remaining serotypes.
Recently, we reported for the first time a recombinant protein for DEN4 (PD24), resulting from the inclusion of the envelope protein domain III in both sites within the P64k in order to increase the viral representation in the chimeric protein (15). This molecule was partially protective in mice after four doses; however, the phenomenon of degradation, previously observed for the insertion variants, affected the yield of the semi-purification process.
The question then arises: which of the alternatives, PD19 or PD24, is the most suitable for developing a DEN4-vaccine candidate based on P64k as carrier? As a first attempt to answer this question, in this study we compared both candidates regarding their molecular and antigenic features, immunogenicity and protective capacity in the viral encephalitis murine model.
MATERIALS AND METHODS
Viruses
A preparation from suckling mice brain infected with DEN4 (H241 strain) was used as antigen for antibody detection (16). A similar preparation obtained from brain of non-inoculated mice was used as negative control. For animal immunization and virus challenge a preparation of infective DEN4 virus (H241 strain) (4.5 × 105 pfu/mL) was employed. It was obtained by homogenization of suckling mice brain infected with DEN4 using the RPMI-1640 medium. For the neutralization assay, clarified cell culture supernatant fluid, harvested from Vero cells infected with DEN4 virus (H241 strain) was used as viral stock. A concentrated preparation of DEN4 (strain H241) was used for in vitro stimulation in the IFNγ detection assay. This preparation was obtained by centrifugation at 80 000 x g, 4 h, 4 ºC of the supernatant from infected Vero cells (100 mL), followed by dissolving into 1 mL of phosphate-buffered saline (PBS). A mock preparation was similarly prepared from the supernatant of uninfected Vero cells.
Recombinant fusion proteins
The recombinant protein PD24 (the DEN4 envelope domain III inserted at two sites within the P64k) was obtained as inclusion bodies in E. coli and was subjected to a denaturation-renaturation procedure (15). After a process of semi-purification the protein reached a purity of 70%. A similar semi-purification process was developed for the recombinant protein PD19 (the DEN4 envelope domain III fused to the C-terminus of the P64k) (8).
SDS-PAGE
Ten micrograms of each sample were loaded per lane and subjected to SDS-PAGE 12.5% as previously described (17). Proteins were solubilized in SDS-PAGE sample buffer containing 1% SDS, 66 mM Tris–HCl, 1% Glycerol, 0.7% Bromophenol Blue and 10 mM β-mercaptoethanol pH 6.8 for 5 min at room temperature. Samples in non-reducing conditions were solubilized in the sample buffer without 10 mM β-mercaptoethanol. The protein bands were visualized by staining with 0.1% Coomassie Brilliant Blue R-250 in 10% acetic acid and 30% methanol.
Mouse immunization
Groups of 22 female Balb/c mice 7-week-old were purchased from CENPALAB (Havana City, Cuba), and housed in appropriate animal care facilities during the experimental period. The animals were handled according to international guidelines for experiments with animals. Mice were immunized by intraperitoneal route on days 0, 15, 30 and 45 with 20 mg of semipurified PD24 or PD19 in 10 mM Tris, 6 mM EDTA adjuvanted in aluminum hydroxide. Similarly, negative control mice received 20 mg of the purified P64k (Placebo group). As positive control one dose of infective DEN4 virus (H241 strain) without adjuvant was administered to other 22 animals. Mice were bled 15 days after the last dose and sera were collected for further immunological analysis.
Enzyme linked immunosorbent assay
An amplified sandwich ELISA system was employed to determine the anti-DEN4 virus antibodies, as previously described (15). For the antigenic characterization we selected four murine and two human sera with IgG titers against DEN4 higher than 1:320 000 and neutralizing titers higher than 1:250.
Plaque reduction neutralization test
Neutralizing antibody titers were measured by the plaque reduction neutralization test (PRNT) using the DEN4 H241 strain with BHK-21 cells, as previously described (18). The neutralization titer was defined as the dilution yielding a 50% reduction in the maximum number of plaque forming units.
Animal protection assay
One month after the last inoculation, mice were injected intracranially with 20 mL of a preparation of infective DEN4 virus containing 50 median lethal doses. Mice were daily observed for 15 days for mortality. The maintenance and care of experimental animals used in this research complied with the international guidelines for the humane use of laboratory animals.
Cell culture
Two months after the last dose, three mice per group were splenectomized in aseptic conditions. The cells were washed twice with 2% fetal bovine sera (FBS) in PBS and resuspended at 2x106 cells/mL in RPMI-1640 medium (Sigma Aldrich, Ayrshire KA, UK) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco), 2 mM glutamine (Glutamax, Gibco), 5x10-5 M 2-mercaptoethanol (Sigma St. Louis, MO) and 5% FBS. The cells (2 x 105 cells/well) were cultured in 96-well round bottom plates with the relevant antigens (dengue virus and mock preparation). Concanavalin A (ConA) (Sigma St. Louis, MO) was used as positive control.
Cytokine detection
The culture supernatants of splenocytes previously stimulated with DEN4 virus were analyzed in duplicate for INFγ-concentration by ELISA using monoclonal antibody pairs (Mabtech (INFγ; Nacía, Sweden). The ELISA protocol recommended by the manufacturers was used with slight modifications. The lowest limit of detection of the cytokine in this assay was 4 pg/mL.
Statistical analysis
The ELISA data were analyzed using a Kruskal Wallis non-parametric test with the Dunn’s Multiple Comparison Test and a Mann Whitney test. The data from the protection assay were analyzed by the Logrank test. In all cases, the software application GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA, http://www.graphpad.com), was employed.
RESULTS
Molecular and antigenic characterization
In order to compare the conformational state of both recombinant proteins after the semipurification pro cess, we performed an SDS-PAGE under reducing and non-reducing conditions (Figure 1). As a result, undernon-reducing conditions, both proteins exhibited the same pattern of aggregation dependent on disulfide bonds, while the carrier protein did not show this behavior despite the six free cysteine residues within its structure (19).
Both recombinant proteins were recognized by murine and human sera (Figure 2). However, protein PD24 showed higher recognition levels by murine and human sera than protein PD19 (p < 0.05 and p < 0.001, respectively). These results indicate a higher antigenicity for PD24.
Immunological evaluation
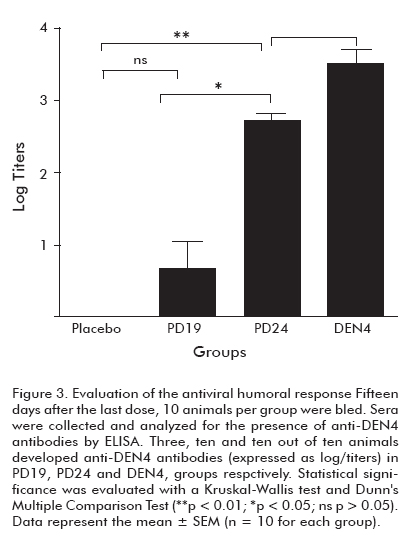
Each semi-purified protein was injected into mice to evaluate the induced immune response. Fifteen days after the fourth dose, 10 animals per group were bled for the analysis of the humoral response (Figure 3). As a result, only 3 mice immunized with PD19 developed antiviral antibody titers, while the animals immunized with PD24 exhibited titers that were statistically similar to those of the positive control group (p > 0.05).
Despite the low levels of antiviral antibodies elicited after the immunization with the recombinant proteins we decided to evaluate the functionality of these antibodies by a plaque-reduction neutralization test against DEN4 virus. None of the sera showed neutralizing activity.
Animal protection assay
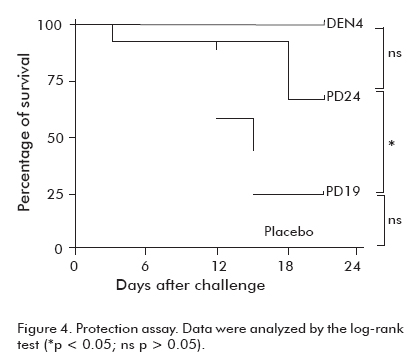
One month after the last dose, animals that were not bled were challenged with neurovirulent DEN4 virus. At the end of the observation period, 67% of animals immunized with PD24 in Al(OH)3 survived, and no statistical differences were observed between this group and the one immunized with the virus (p > 0.05). Only 3 mice survived from those immunized with PD19, whereas 100% of the animals from the group immunized with the virus were protected (Figure 4).
Detection of IFNγ secretion
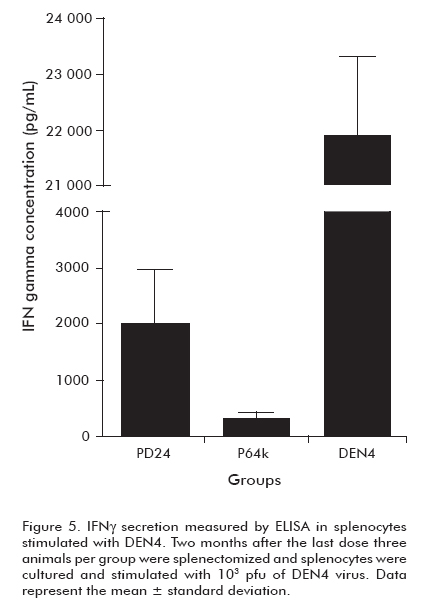
Regarding the lack of functional humoral response induced by the protein PD24 and the significant protection it afforded upon viral challenge, we performed an assay to determine the elicited cell-mediated immune response.
Two months after the last dose, animals immunized with P64k, PD24 and VD4 were sacrificed. Splenocytes from three mice per group were stimulated in vitro with infective DEN4 virus. After four days of incubation, culture supernatants were harvested and analyzed for mouse IFNγ using an (Figure 3). Evaluation of the antiviral humoral response.
Fifteen days after the last dose, 10 animals per group were bled. Sera were collected and analyzed for the presence of anti-DEN4 antibodies by ELISA. Statistical significance was evaluated with a Kruskal Wallis test and Dunn’s Multiple Comparison Test (**p < 0.01; *p < 0.05; ns p > 0.05). Data represent the mean ± SEM (n=10 for each group).
ELISA
As a result, the splenocytes from the group of mice immunized with protein PD24 secreted higher levels of IFNγ than those from the group immunized with the protein carrier (Figure 5).
DISCUSSION
In a previous work we suggested that the insertion of two protein fragments within the P64k induced, in the final recombinant protein, an aggregation pattern dependent on disulfide bonds formation (15). Results from the present study demonstrate that the cysteine-mediated multimerization observed in PD24 is not a consequence of the insertion of an additional domain III within the LBD of P64k. The fusion of DEN4 envelope protein domain III to the C-terminus of P64k induced the same phenomenon. Dissimilar results were obtained with the recombinant protein (fusion variant) corresponding to serotype 2 (PD5) (20, 21). In this case the aggregation pattern was avoided em-ploying the Zn2+ ion in the immobilized-metal-ion affinity-chromatography (IMAC) (20). Considering that the semipurification process performed in our study was similar to that reported for PD5 (20), we can speculate that some intrinsic feature of the DEN4 envelope domain III may lead to an improper conformation of PD19 and PD24 after the refolding process. However, both recombinant proteins were recognized by murine and human sera, suggesting the correct con-formation of the DEN4 envelope domain III in the final molecule.
Despite similarities in the molecular characterization of PD19 and PD24, only the last one protected mice from the lethal challenge with DEN4. Consistently, PD24 exhibited the highest antiviral response; however, none of the sera showed neutralizing capacity.
In 2007, Volk et al. reported differences between the electrostatic charge on the surface of the DEN4-domain III and the other serotypes, and demonstrated differences between their antigenicity employing two DEN complex-reactive MAbs (22). In agreement, some studies have reported low immunogenicity for DEN4-domain III in tetravalent formulations (2). Additionally, based on several epidemiological and immunological data, DEN4 has been described as a naturally attenuated virus (23). Therefore, the lack of neutralizing antibodies in our study might have arisen from the aggregated nature of the protein PD24 and the low antigenicity of the DEN4 virus. In fact, mice immunized with the infective virus did not elicit neutralizing antibodies.
Nevertheless, protein PD24 induced antiviral antibodies. It is well known that the envelope protein is expressed on the surface of infected cells (24). Therefore the antibody-dependent cellular cytotoxicity, mediated by the antibodies elicited against the domain III, might be one of the mechanisms of survival involved.
On the other hand, the cell-mediated immunity may be playing an important role in the results achieved in our study. There are several studies demonstrating the role of the cellular immune response in protecting against dengue virus in the mouse encephalitis model (25-27). The levels of IFNγ secretion in mice immunized with PD24 support this hypothesis. Accordingly, previous studies have found a relationship between CD8 cytotoxic activity and IFNγ secretion (27, 28).
Our study endorses the strategy of using both sites of inclusion within the same P64k molecule, in order to enhance the immunogenicity of protein fragments. Subsequent studies assessing tetravalent formulations will include the protein PD24 as the DEN4 vaccine candidate.
REFERENCES
1. Zulueta A, Martin J, Hermida L, Alvarez M, Valdes I, Prado I,, et al. Amino acid changes in the recombinant Dengue 3 Envelope domain III determine its antigenicity and immunogenicity in mice. Virus Res 2006;121:65-73.
2. Simmons M, Murphy GS, Hayes CG. Short report: Antibody responses of mice immunized with a tetravalent dengue recombinant protein subunit vaccine. Am J Trop Med Hyg 2001;65:159-61.
3. Srivastava AK, Putnak JR, Warren RL, Hoke CH, Jr. Mice immunized with a dengue type 2 virus E and NS1 fusion protein made in Escherichia coli are protected against lethal dengue virus infection. Vaccine 1995;13:1251-8.
4. Fonseca BA, Khoshnood K, Shope RE, Mason PW. Flavivirus type-specific antigens produced from fusions of a portion of the E protein gene with the Escherichia coli trpE gene. Am J Trop Med Hyg 1991;44:500-8.
5. Hermida L, Rodriguez R, Lazo L, Silva R, Zulueta A, Chinea G,, et al. A dengue-2 Envelope fragment inserted within the structure of the P64k meningococcal protein carrier enables a functional immune response against the virus in mice. J Virol Methods 2004;115:41-9.
6. Hermida L, Rodriguez R, Lazo L, Bernar-do L, Silva R, Zulueta A, et al. A fragmentof the envelope protein from dengue-1virus, fused in two different sites of the meningococcal P64k protein carrier, induces a functional immune response in mice. Bio-technol Appl Biochem 2004;39:107-14.
7. Hermida L, Bernardo L, Martin J, Alvarez M, Prado I, Lopez C, et al. A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine 2006;24:3165-71.
8. Zulueta A, Hermida L, Lazo L, Valdes I, Rodriguez R, Lopez C, et al. The fusion site of envelope fragments from each serotype of Dengue virus in the P64k protein, influence some parameters of the resulting chimeric constructs. Biochem Biophys Res Commun 2003;308:619-26.
9. Li de lS, I, Pernot L, Prange T, Saludjian P,Schiltz M, Fourme R, et al. Molecular structure of the lipoamide dehydrogenase domain of a surface antigen from Neisseria meningitidis. J Mol Biol 1997;269:129-41.
10. Pérez AE, Dickinson FO, Banderas F, Serrano T, Llanes R, Guzman D, et al. Safety and preliminary immunogenicity of MenC/P64k, a meningococcal serogroup C conjugate vaccine with a new recombinant carrier. FEMS Immunol Med Microbiol 2006;46:386-92.
11. González S, Alvarez A, Caballero E, Vina L, Guillén G, Silva R. P64k meningococcal protein as immunological carrier for weak immunogens. Scand J Immunol 2000;52:113-16.
12. Carmenate T, Canaan L, Alvarez A, Delgado M, González S, Menéndez T, et al. Effect of conjugation methodology on the immunogenicity and protective efficacy of meningococcal group C polysaccharide-P64k protein conjugates. FEMS Immunol Med Microbiol 2004;40:193-99.
13. Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, et al. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am J Trop Med Hyg 2002;66:264-72.
14. Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, et al. Safety and immunogenicity of a three dose regimen of two tetravalent live-attenuated dengue vaccines in five- to twelve-year-old Thai children. Pediatr Infect Dis J 2004;23:99-109.
15. Lazo L, Zulueta A, Hermida L, Blanco A, Sánchez J, Valdés I, et al. Dengue-4 envelope domain III fused twice within the meningococcal P64k protein carrier induces partial protection in mice. Biotechnol Appl Biochem 2009;52:265-71.
16. Clarke DH and Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg 1958;7:561-73.
17. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-685.
18. Morens DM, Halstead SB, Repik PM, Putvatana R, Raybourne N. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J Clin Microbiol 1985;22:250-54.
19. Guillén G, Alvarez A, Silva R, Morera V, González S, Musacchio A, et al. Expression in Escherichia coli of the lpdA gene, protein sequence analysis and immunological characterization of the P64k protein from Neisseria meningitidis. Biotechnol Appl Biochem 1998;27 (Pt 3):189-96.
20. López C, Sánchez J, Hermida L, Zulueta A, Márquez G. Cysteine mediated multimerization of a recombinant dengue E fragment fused to the P64k protein following immobilized metal ion affinity chromatography. Protein Expr Purif 2004; 34:176-82.
21. Valdés I, Hermida L, Martín J, Menéndez T, Gil L, Lazo L, et al. Immunological evaluation in nonhuman primates of formulations based on the chimeric protein P64k-domain III of dengue 2 and two components of Neisseria meningitidis. Vaccine 2009;27:995-1001.
22. Volk DE, Lee YC, Li X, Thiviyanathan V, Gromowski GD, Li L, et al. Solution structure of the envelope protein domain III of dengue-4 virus. Virology 2007;364:147-54.
23. Vaughn DW. Invited commentary: Dengue lessons from Cuba. Am J Epidemiol 2000;152:800-3.
24. Halstead, S. (2008) Dengue, Imperial College Press, London.
25. Gil L, López C, Blanco A, Lazo L, Martín J, Valdés I, et al. The cellular immune response plays an important role in protecting against dengue virus in the mouse encephalitis model. Viral Immunol 2009;22:23-30.
26. Lazo L, Hermida L, Zulueta A, Sánchez J, López C, Silva R, et al. A recombinant capsid protein from Dengue-2 induces protection in mice against homologous virus. Vaccine 2007;25:1064-70.
27. Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, et al. A protective role for dengue virus-specific CD8+ T cells. J Immunol 2009;182:4865-73.
28. van der Most RG, Murali-Krishna K, Ahmed R. Prolonged presence of effector-memory CD8 T cells in the central nervous system after dengue virus encephalitis. Int Immunol 2003;15:119-125.
Received in September, 2009.
Accepted for publication in December, 2009.
Laura Lazo. Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 / 158 and 190, Playa, PO Box 6162, Havana, Cuba. E-mail: laura.lazo@cigb.edu.cu