My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.26 no.4 La Habana Oct.-Dec. 2009
REPORT
CIMAvax-EGF: A novel therapeutic vaccine for advanced lung cancer
Gisela González1, Agustin Lage1, Tania Crombet1, Gryssel Rodríguez1, Beatriz García1, Ariadna Cuevas1, Lisel Viña1, Norkis Arteaga1, Elia Neninger2
1Center of Molecular Immunology, CIMAve. 216 corner 15, Atabey, Playa, POBox 16 040, Havana, Cuba
2Hermanos Ameijeiras HospitalSan Lázaro #701 e/ Belascoín y Marqués González, Centro Habana, Ciudad de La Habana, Cuba.
ABSTRACT
The results allowing the Cuban Regulatory Agency (CECMED) to grant the Sanitary Registration to the CIMAvax-EGF cancer vaccine for advanced non-small cell lung cancer (NSCLC) are shown. This was the first registration of a therapeutic vaccine in Cuba and also the first registration of a lung cancer vaccine in the world. Hence, a unique therapeutic vaccine is offered to lung cancer patients, which will increase survival and their quality of life. For this purpose, significant preclinical, clinical, regulatory, productive and negotiation challenges were to be faced. The results obtained in these fields led to 18 scientific papers published in high impact journals and 4 invention objects, generating several patents in Cuba and other countries. In the pre-clinical setting, immunogenicity, safety and anti-tumoral effects were demonstrated in different animal species. The clinical experience began in 1995. Up to now, five phase I-II clinical trials have concluded in Cuba, two phase II have also concluded, one in Cuba and another one in Canada and the UK, and a phase II-III trial with an optimized schedule as well as a phase III trial are currently in progress in Cuba. In the regulatory field, a fast-track registration strategy was designed and performed. It required novel regulatory conceptions to develop this unique product. A scalable, reproducible and controlled productive process was carried out, together with a quality system that ensured full GMP compliance. Funds for product development came from implementing a novel negotiation strategy: negotiation of intangibles.
Keywords: Cancer vaccine, NSCLC, Fast-Track Registration, CIMAvax-EGF, Phase II-III Clinical Trial
INTRODUCTION
The relationship between the system formed by the Epidermal Growth Factor receptor (EGFR) and its ligands with cancer development is well known. In epidermoid origin tumors, there is an over-expression of the EGFR that relates to bad prognoses and early relapses after surgery. That is why this system has become an important target for anti-tumor therapies.
Cell proliferation mechanisms are initiated with the binding of EGF to EGFR. Our therapeutic approach consists of a vaccine with an EGF formulation making it immunogenic and inducing a humoral immune response. The production of specific anti-EGF antibodies that bind to the autologous EGF, prevents it from binding to the EGFR thereby triggering the cell proliferation mechanisms derived from that interaction (1-4).
Here we report the results of the registration in Cuba of this vaccine (CIMAvax-EGF). This was part of a global strategy comprising novel clinical, regulatory, and technological and business contributions, and is supported by 18 international scientific papers and intellectual property worldwide. We also describe innovations in different fields that enabled the registration of CIMAvax-EGF in Cuba and Peru.
RESULTS AND DISCUSSION
Vaccination with EGF is safe, immunogenic and increases survival with a good quality of life in patients at advanced stages lung cancer
The clinical experience with CIMAvax-EGF in the therapeutics of non-small-cell lung cancer (NSCLC) at advanced stages began in 1995. Up to now, five phase I-II clinical trials have been concluded in Cuba; 2 randomized phase II clinical trials were concluded, one in Cuba and another one in Canada and the UK, and there is a phase III trial in progress in Cuba. More than 800 advanced cancer patients have been treated with CIMAvax–EGF, thereby demonstrating it to be safe, immunogenic and able to increase survival with a good quality of life.
The main objective of the phase I-II trials was to decide the best vaccine formulation (carrier protein and adjuvant), the dose and the therapeutic schedule. These results demonstrated the advantages of the P64k protein as the carrier protein and Montanide ISA51 as the adjuvant. The increased immunogenicity of scaling-up the dose was also demonstrated (5-7).
The analysis of the pooled data from all the phase I-II trials showed a significant increase in survival in those patients with better antibody responses or good antibody responders (GAR), and in patients with more pronounced decreases in sera EGF concentrations ([EGF]) resulting from vaccination. A significant increase in survival of all vaccinated patients was also demonstrated when compared with a concurrent historical control (7).
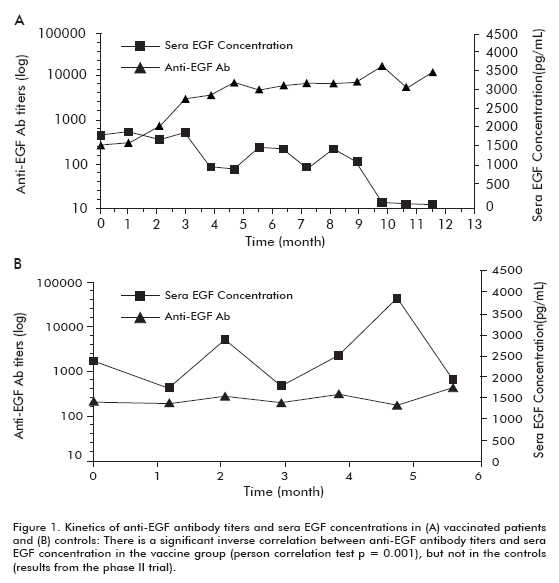
A phase II clinical trial was performed in 80 patients, who were randomized to receive the CIMAvax-EGF and the best supportive care (BSC) (40 patients) or BSC alone (40 patients), after concluding first line chemotherapy. The previous findings were corroborated in this trial. Approximately 50% of the vaccinated patients were GAR and survived significantly more than patients who did not reach the GAR classification (who were classified as poor antibody responders, PAR). Similarly, patients with the greatest decrease in [EGF] survived significantly more than patient who did not show this decrease (Table 1). A significant inverse correlation was observed between anti-EGF antibody titers and [EGF], which occurred in vaccinated patients but not in the controls, thus demonstrating that it was caused by vaccination (Figure 1).
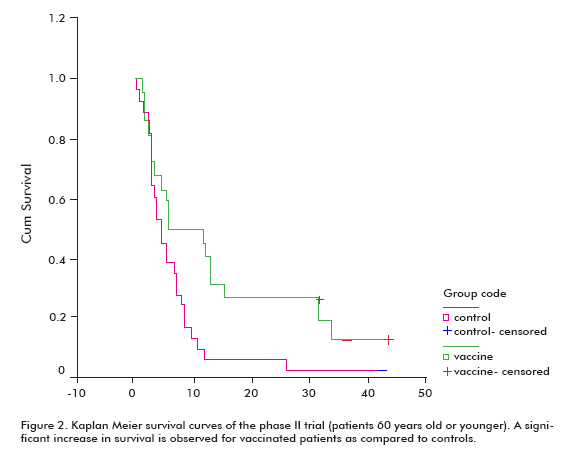
There was an increased trend in survival in all vaccinated patients (18.53 ± 11.47; mean ± median), compared with the non-vaccinated controls (7.55 ± 5.33), which was significant in the group of patients that were 60 years old or younger (Log rank test; p < 0.05) (Figure 2) (8).
As evidenced in an in vitro competition assay, sera from vaccinated patients inhibited the binding of EGF to EGFR. This binding inhibition was proportional to antibody titers and survival. Additionally, sera from vaccinated patients inhibited EGFR phosphorylation in proportion to its anti-EGF antibody titers.
The preferential recognition of the EGF/EGFR binding site (loop B of the EGF molecule) was studied. Patients whose sera preferentially recognize loop B survived significantly more than patients who did not recognize this epitope (9).
A dose/schedule optimization trial was recently concluded in Cuba (phase I-II). In its design, all elements increasing immunogenicity in previous trials were considered, which included: Montanide ISA51 as the adjuvant, increased doses, four injection sites and administration of two vaccine doses prior to first line chemotherapy, followed by vaccination. Results from this trial demonstrated a significant increase in immunogenicity, with a 95% of GAR reaching ten times the previous maximal antibody titers. All vaccinated pa-tients survived significantly more than controls from the phase II trial (10).
An increased capacity of the sera of patients to inhibit EGF/EGFR binding and EGFR phosphorylation was also found. On month seven, after concluding chemotherapy, the antibody response shifted towards loop B of the EGF molecule, which could indicate an improvement in the quality of the immune response. Results from this trial demonstrated that there is a margin of improvement in the response to CIMAvax-EGF that can be reached by manipulating the dose and the therapeutic schedule (10).
The results of these trials are being validated in a phase III trial currently in progress in Cuba.
Design and implementation of a regulatory strategy for vaccine registration to make it available to all advanced non-small-cell lung cancer patients
CIMAvax-EGF is a therapeutic vaccine for lung cancer that is unique in the world. A global strategy was designed and implemented; it was guided by regulatory requirements, for fast track registration in Cuba. A close relationship between the sponsor (CIM) and the Cuban Regulatory Agency (CECMED) had the purpose of establishing the requirements for obtaining a product and its fast application in the benefit of advanced cancer patients without any other therapeutic alternative. This strategy included the design of a quality system, quality controls and specifications, as well as GMP requirements to cover the different steps of product development. This global strategy can be used in the development of other similar products.
The accumulated regulatory experience and documentation led to the approval of clinical trials in different countries. The dossier containing all the information made it possible to register CIMAvax-EGF in Cuba and Peru. We can therefore state that we have a defined strategy for drug registration in different countries.
The GMP production process that generates a product that is scalable, consistent and complies with specifications
The first challenge to design a production process for CIMAvax-EGF was to have an immunogenic EGF preparation. It was obtained by the chemical conjugation of the human recombinant EGF to the recombinant membrane protein, P64k (from Neisseria meningitidis). The conjugate is injected together with an appropriate adjuvant (currently, Montanide ISA 51 from Seppic, France).
A process at a laboratory scale was initially designed. A scale-up (and scalable) sanitary and reproducible process was later designed and implemented, complying with GMP guidelines. In vitro and in vivo analytical assays were also designed and implemented to evaluate the quality of the raw materials, and the intermediate and final products.
All modifications were assessed and supported by the current Regulatory strategy. Results from the equivalence study, to compare both products, received the approval by CECMED of the scaled process application, and the approval of the product obtained in this process for its clinical use. The scaling-up and optimization of the production process gave way to a publication (11) and a new invention object, with patents subsequently presented in different countries.
Negotiation of intangibles granted us funds for project development and also learning experience in our joint work with regulatory authorities from different countries
This Project was negotiated with foreign counterparts on the basis of the novel concept of “negotiation of intangibles”, supported mainly through the intellectual property of our product that was under development. This negotiation strategy consisted of licensing the project for its joint development with other countries, which means that the foreign counterparts covered the expenses of regulatory actions and clinical trials in their territories. As a result of these negotiations, Cuba received payments for milestones. This modality for negotiation also allowed us to have a constant exchange with specialists here and abroad, thereby increasing our experience. Our positive results with this negotiation modality could be applied to other products in progress in our country.
RELEVANCE OF THE STUDY
CIMAvax-EGF is the first therapeutic vaccine for cancer treatment registered in Cuba, and the first registered in the world for lung cancer therapy. Lung cancer is an unsolved health problem with more than 1.3 million cases diagnosed every year and the same number of deaths throughout the world. In Cuba, it is the main cause of death due to cancer in both sexes with an incidence of more than 4000 deceased per year. The therapy of choice for patients diagnosed at advanced stages is that of first line chemotherapy, which can be administered concurrently with radiotherapy. This is a palliative, non curative treatment, with complete response to chemotherapy being very rare, and objective responses (complete or partial) occurring in only 25% of the patients.
CIMAvax-EGF offers an alternative treatment for these patients that have already received first line chemotherapy. The severe adverse events of chemotherapy and/or radiotherapy treatments are well known, and severely deteriorate quality of life. Instead, vaccination with CIMAvax-EGF generates only mild or moderate, rather than severe, adverse events that can be solved with conventional supportive care, while improving the survival of advanced lung cancer patients and offering a good quality of life.
Until now, more than 800 Cubans have been treated with CIMAvax-EGF in more than 20 hospitals throughout the country. The Registration of CIMAvax-EGF allows it to be extended to all the patients in the country who were diagnosed with advanced lung cancer; thus making Cuba the first country in the world with a national program using this therapeutic alternative.
Regarding economic benefits, through the negotiations of this Project we have obtained 6.346 million USD. The negotiation strategy of the project is for its joint development with other countries, which means that the foreign counterpart covers the expenses of the clinical trials in their own country, making it affordable. These trials require investments of millions of USD, and this is only possible if it is supported by foreign counterparts. At the same time, this strategy offers more clinical data to Cuba, without the expenses of carrying out the trials here. Until now, clinical trials have been performed in Canada, UK and Malaysia. In the near future, clinical trials will begin in China and Europe.
Also, as a part of this licensing strategy, the foreign counterparts are in charge of product registration in the regions where they have commercial rights. During the registration and marketing processes, Cuba will receive payments for milestones and later, as sales royalties.
The world market of cancer vaccines is considered to be in the order of billions of USD. The fact that Cuba has a unique product for advanced lung cancer treatment can provide us with an export potential that may fund the use of this product in our National Health System.
CONCLUSIONS
Vaccination with CIMAvax-EGF is safe, immunogenic and leads to an increase in survival with a good quality of life in patients with lung tumors at advanced stages. The design and implementation of a regulatory strategy for registering this vaccine made it available
for all cases of advanced non-small cell lung cancer patients in Cuba. This positive experience could be applicable to other similar products in progress. A production process was developed, which was scalable, consistent, and reproducible and complied with GMP guidelines, to generate a product that complies with specifications. The experience in negotiation of intangibles has been very positive, and has granted us funds for project development, while supporting the learning experience derived from joint work with the regulatory authorities of different countries.
ACKNOWLEDGEMENTS
The authors want to thank to the following specialists from the Center of Molecular Immunology for their con-tribution to this work: Rolando Pérez, Loany Calvo, Suhamy Atencio, Belinda Sánchez, Irene Beausoleil, Ernesto Chico, Airama Alvisa, Ileana Cartroman, Sergio Cata, Ana Veloso, Reinaldo Cuervo, Yanelda López, Yosniel Hernández, Antonio Vallin, Alejandro Portillo, Liuva Madera, Guido Ferrer, Diana Borges, Niuvis Pérez, Tamara García, Idaine Cuenca, Mayra Santaelena, Joaquin Solozabal, Aida Rodríguez, María Elena García, Adis Torres, Eric Chong, Carmen Viada, Mauricio Catala, Soraida Acosta, Bárbara Wilkinson, Olga Torres, Normando Iznaga all of them from the Center of Molecular Immunology, and also to Daniel González, Lourdes B Costa, Galina M Moya, Rolando Páez, Gerardo Guillén, Vivian Pujol , Dinorah Torres from the Center for Genetic Engineering and Biotechnology.
REFERENCES
1. González G, Montero E, León K, Cohen IR, Lage A. Autoimmunization to Epidermal Growth Factor, a component of the im-munological homunculus. Autoimmun Rev 2002;1:89-95.
2. Lage A, Crombet T, González G. Targeting epidermal growth factor receptor signalling: early results and future trends in oncology. Ann Med 2003;5(35):327-36.
3. González G and Lage A. Cancer Vaccines for Hormone Immune-Deprivation: The EGF Vaccine Approach: Leading Topics in Cancer Research, Chapter 11, Ed Nova Publishers, 2007.
4. González G and Lage A. Cancer vaccines for hormone/growth factor immune deprivation: a feasible approach for cancer treatment. Curr Cancer Drug Targets 2007;7:229-41.
5. González G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M, et al. Epidermal Growth Factor-based cancer vaccine for non-small cell lung cancer therapy. Ann Oncol 2003;14:461-6.
6. Crombet T, Neninger E, Catalá M, García B, Leonard I, Martínez L, et al. Treatment of NSCLC patients with an EGF based cancer vaccine. Report of a phase I trial. Cancer Biol Ther 2006;5(2):136-41.
7. González G, Crombet T, Neninger E, Viada C, Lage A. Therapeutic vaccination with epidermal growth factor (EGF) in advanced lung cancer: analysis of pooled data from three clinical trials. Hum Vaccines 2007;3(1):8-13.
8. Neninger E, De la Torre A, Osorio M, Catald M, Bravo I, Mendoza M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol 2008;26:1452-8.
9. García B, Neninger E, De la Torre A, Leonard I, Martínez R, Viada C, et al. Effective inhibition of the Epidermal Growth Factor/Epidermal Growth Factor Receptor binding by anti-Epidermal Growth Factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the Epidermal Growth Factor vaccine. Clin Cancer Res 2008; 14(3):840-6.
10. Neninger E, Verdecia BG, Crombet T, Viada C, Pereda S, Leonard I, et al. Combining an EGF-based cancer vaccine with chemotherapy in advanced non small cell lung cancer. J Immunother 2009;32:92-9.
11. Rodríguez G, Albisa A, Viña L, Cuevas A, García B, García AT, et al. Manufacturing process development for an epidermal growth factor based cancer vaccine. Bio-pharm Int, Vaccines Suppl, Oct 2008.
Gisela González. Center of Molecular Immunology, CIMAve. 216 corner 15, Atabey, Playa, POBox 16 040, Havana, Cuba. E-mail: gisela@cim.sld.cu