Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.27 n.2 La Habana abr.-jun. 2010
RESEARCH
Impact of epidermal growth factor on the treatment of diabetic foot ulcers
Impacto del Heberprot-P en el tratamiento de las úlceras del pie diabético
Wilver Velázquez, Alfredo Valles, Walfrido Curbelo
Hospital General Docente "Guillermo Domínguez López" Puerto Padre, Las Tunas, Cuba
ABSTRACT
Human Epidermal Growth Factor (hEGF), obtained through recombinant DNA technology and formulated as Heberprot-P at a dosage of 75 µg, can stimulate the granulation and cicatrization of different tissues. This work is aimed mainly at the evaluation of the impact of the intralesional administration of hEGF in diabetic foot ulcers (DFU) treated with the optimized insulin scheme, in terms of whether it facilitates granulation and cicatrization or decreases the number of amputations. An evaluative, longitudinal intervention method was applied in 32 patients with a diagnosis of Diabetic Foot that complied with the inclusion criteria (Wagner grade 3 and 4 ulcers, patient older than 18 years, signed informed consent form), excluding cases where ulcer area was smaller than 1 cm2 or there were decompensated chronic diseases: diabetic coma, ischemic cardiopathy, CRI (creatinine > 200 mmol/L, oligoanuria and antecedents or suspicions of malignant disease) and applying the treatment intralesionally, thrice per week. Both granulation and cicatrization of the lesions were achieved in 90.62% of the cases in an average time of 46.5 days ± 8.9. Amputation was necessary only in 9.38% of the cases. The most frequent adverse events were pain, burning sensations, and shivering. The therapeutic scheme of intralesional administration of Heberprot-P can complete lesion closure and is a convenient and safe alternative in the treatment of advanced diabetic foot ulcers, constituting a unique therapeutic modality for a critical and incapacitating disease.
Keywords: diabetic foot ulcer, Heberprot-P, Wagner classification
RESUMEN
El factor de crecimiento epidérmico humano (FCEH) obtenido con tecnología recombinante en su formulación Heberprot-P de 75 µg puede estimular la granulación y cicatrización en distintos tejidos. El objetivo fundamental de este trabajo investigativo fue evaluar el impacto de la administración intralesional del FCEH en úlceras de pie diabético (UPD), en pacientes tratados con esquema optimizado de insulina, para comprobar si facilitaba la granulación y cicatrización; y si al mismo tiempo disminuían las amputaciones. Se utilizó un método de intervención, evaluativo, longitudinal y prospectivo en 32 pacientes con diagnóstico de pie diabético, con criterios de inclusión (úlceras de grado 3 y 4, según la clasificación de Wagner, mayor de 18 años y voluntariedad del paciente con la firma del consentimiento informado). Se excluyeron los casos con úlceras con un área menor que 1 cm2, enfermedades crónicas descompensadas: coma diabético, cardiopatía isquémica, IRCr (creatinina > 200 mmol/L, oligoanuria, y antecedentes o sospecha de enfermedades malignas. El Heberprot-P se aplicó 3 veces por semana de forma intralesional. La granulación como la cicatrización de las lesiones se lograron en el 90.62%, en un término promedio de 46.5 días ± 8.9. La amputación solo fue necesaria en el 9.38% de los casos. Los eventos adversos más frecuente fueron el dolor, ardor y tiriteo. El esquema terapéutico de la administración intralesional del Heberprot-P puede completar el cierre, ser seguro y conveniente para sanar las úlceras del pie diabético avanzado. Representa una modalidad terapéutica singular para enfermedades críticas y discapacitantes.
Palabras clave: úlcera de pie diabético, Heberprot-P, clasificación de Wagner
INTRODUCTION
Diabetes is the main non-traumatic risk factor for the amputation of lower limbs. It has been estimated that close to 1000 such amputations take place in our country each year. Therefore, wound cicatrization problems not only have a high clinical relevance, but their economic cost reaches thousands of millions of dollars per year (1).
Diabetic Foot Ulcer (DFU) is a significant complication of Diabetes Mellitus (DM) with a yearly incidence of 10% among diabetic patients, further increased by 5 to 7.5% in those suffering from peripheral neuropathies. It is estimated that 15% of diabetic patients develop ulcers at some point during their life, and 10 to 30% of these ulcers eventually progress to an amputation. The presence of infections is an important contributing factor for this event, as according to the literature, approximately 60% of these amputations are preceded by the presence of infected ulcers. The mortality after 5 years in patients undergoing a lower limb amputation is 50 to 60% (1, 2).
DFU is one of the most common disorders related to the appearance of problems in the process of cicatrization. There is a high incidence of systemic endothelial disorders in diabetic patients, who are saddled with weakened anti-bacterial defenses and a deteriorated machinery for tissue repair; precisely, the combination of these factors results in a higher incidence of lower limb amputations in this clinical population. The cornerstones of wound treatment in diabetics are a meticulous lesion management, and the stimulation of revascularization whenever feasible. However, these interventions are often not effective, and an amputation becomes unavoidable (3-6).
Tissue repair requires the coordinated interaction of numerous cell types in processes of inflammation; matrix deposition and remodeling that restore the continuity and architecture of visceral or cutaneous defects (7, 8). Precisely, the epidermal growth factors (EGF) constitute the largest population of soluble messengers that fine-tune and regulate the complex network of processes that take place during tissue repair. The mechanisms whereby DM obstructs tissue repair are still under study (9), but a set of convergent evidences suggests a deficit in the production of different EGF, such as Keratinocyte Growth Factor (KGF), Vascular Endothelium Growth Factor (VEGF) and Platelet-Derived Growth Factor (PDGF) as one of the main culprits (10, 11).
The EGF receptor has been identified as a target for Advanced Glycation End Products (AGEs) such as glyoxal (GO) and methylglyoxal (MGO) in a timeand dose-dependent fashion, resulting in the suppression of receptor self-phosphorylation and of its subsequent activation. The formerly described deficit of EGF in Diabetes: (1) affects fibroblast functionality, limiting the formation, maturation and remodeling of the extracellular matrix; (2) decreases the size of myo-fibroblast populations, leading to an insignificant contraction of the wound; (3) produces a limited or failed angiogenic response. The convergence of these factors leads to a phenotype of wound chronification with a significant impairment of the cicatrization process (4, 6, 12).
EGF stimulates the proliferation of fibroblasts, keratinocytes and endothelial cells in blood vessels, which contributes to its beneficial effects on cicatrization (13). Previous Phase I and II trials in DFU patients have demonstrated that the intralesional administration of Heberprot-P 75 µg (hEGF) stimulates cicatrization, resulting in the formation of useful granulation tissue in the ulcer bed that allows secondintention healing or the successful use of skin grafts, and these effects are associated to a reduction of the risk of amputation (4). These results support the introduction of the exogenous administration of growth factors as an instrumental therapy for the improvement and support of the cicatrization process in this specific patient population. Growth factor “replacement therapy” has so far included the topical release of recombinant human EGF (5).
The risk of a minor or major amputation in a lower limb is greatly increased in diabetics compared to the normal population (14). A large number of studies shows that the cumulative incidence of amputations in the population of patients diagnosed with DM before 30 years of age and an evolution of the disease lasting more than 10 years is higher than 5% and 7% for type-I and type-II DM, respectively (15, 16). When a diabetic patient develops a foot ulcer, the chances of trouble-free cicatrization are small to begin with, compounded by a higher probability of infection, and also higher probabilities of spreading of the infection, leading to a gangrene that finally necessitates amputation (17).
Although different preventive, medical and surgical treatment models have been tried for the management of DFU, a significant decrease of the amputation rate is yet to be accomplished, especially in patients suffering from Wagner’s grade 3 (deep, with abscesses and osteomyelitis) and grade 4 (delimited gangrene of the toe or the foot) ulcers, and the quest for alternatives that come closer to this goal is still ongoing. In this sense, the particularly severe deficit of EGF in DM offers a window of opportunity for a therapy that can result in a significant improvement of the quality of life of these patients (18).
Heberprot-P 75 µg, a new product from Cuban biotechnology obtained in the Center for Genetic Engineering and Biotechnology through recombinant DNA technology, is a preparation of hEGF purified from engineered cells of Saccharomyces cerevisiae bearing a copy of the human EGF gene. Its availability will facilitate the application of doses of 75 µg in three weekly sessions following the intralesional route, until granulation or significant cicatrization is achieved, for a treatment period of up to 8 weeks.
Heberprot-P 75 µg is an injectable cytoprotective agent that stimulates cicatrization, presented as a lyophilizate in glass vials, to be administered intralesionally. This formulation led us to propose a hypothesis.
Can Heberprot-P 75 µg solve the existing contradiction between the EGF deficit of the diabetic and the decrease or absence of granulation and cicatrization of Diabetic Foot Ulcers, while also decreasing the rate of lower limb amputations at the “Guillermo Domínguez López” General Teaching Hospital at the municipality of Puerto Padre, in the province of Las Tunas?
The opportunity to use this drug in our facilities for the treatment of Wagner grade 3 and 4 ulcers will let us validate the impact of this therapy, simultaneously examining the behavior of the variables of study (age, sex, type of diabetes, optimized insulin treatment and Wagner grade of the ulcer), providing an account of any adverse events suffered by the patients, and determining the efficacy of Heberprot-P 75 µg by measuring the proportion of patients achieving complete closure and/or complete granulation of the lesion by the end of the treatment, also evaluating whether there is a reduction in the rate of amputations.
MATERIALS AND METHODS
To evaluate the effect of the intralesional administration of the Heberprot-P 75 µg formulation of hEGF into Diabetic Foot Ulcers, assessing whether it stimulates the granulation and cicatrization of DFU that comply with amputation criteria, and evaluating whether the treatment results in the elimination of such outcome.
Sample: 32 patients with a diagnosis of Wagner grade 3 and 4 diabetic foot ulcers.
Inclusion criteria: Wagner grade 3 and 4 diabetic foot ulcers, age older than 18 years, voluntary participation as attested by signed informed consent form.
Exclusion criteria: Ulcer area < 1 cm2, decompensated chronic diseases: diabetic coma, ischemic cardiopathy, CRI (creatinine > 200mmol/l, oligoanuria and antecedents or suspicions of malignant disease).
Posology and mode of administration: Heberprot-P was used always in conjunction with best practices for the management of diabetic foot ulcers, debriding the lesions whenever necessary, relieving pressure zones, and systematically cleaning, disinfecting and dressing the wounds. It was administered in doses of 75 µg, dissolved into 5 mL of water for injection, 3 times per week, intralesionally. The administrations were continued until the complete granulation of the lesion, its closure via skin grafts, or once the upper limit of the treatment period was met. The infiltrations were performed with a 26 G x ½” needle after cleaning the lesions, infiltrating the drug into their edges, or in the bottom in the case of deeper lesions. The cleanest areas of the lesions were infiltrated first, changing the needle between puncture sites to avoid spreading any existing sepsis and covering later the lesion with gauze wetted with saline solution so as to maintain a moist and clean environment. The evaluation took into account the cases failing to produce useful granulation tissue covering the complete extension of the ulcer, also evaluating the treatment and other factors that might hamper cicatrization such as osteomyelitis, local infections and metabolic disorder.
Study variables: Age, sex, type of diabetes, optimized control with insulin, Wagner classification of the diabetic foot, adverse events, proportion of patients achieving cicatrization or granulation of the lesion, and amputees.
Statistical processing: Medical record, personal file or form, informed consent.
The statistical processing of all collected data was performed manually with a calculator and a Pentium III personal computer under the Windows XP operating system, performing word and table processing with Word XP.
Objectives:
To identify control variables in each patient: age, sex, type of diabetes, Wagner’s diabetic foot classification.
To identify and characterize the adverse events appearing in patients treated with Heberprot-P 75 µg.
To determine the efficacy of Heberprot-P 75 µg by measuring the proportion of patients achieving full closure and/or complete granulation of the lesion by the end of the treatment period.
To evaluate the impact of the treatment in the reduction of the amputation rate.
RESULTS AND DISCUSSION
In our judgment, age is a non-vulnerable risk factor in the vascular complications of Diabetes Mellitus, and it is directly proportional to the number and severity of complications (Table 1). The patients older than 70 years comprised 49.99% of the sample, therefore constituting the largest age group of the sample in agreement with the results of other authors (1, 9, 10) uncovering a higher incidence of peripheral vascular diseases after the 7th decade of life, with an increasing trend. In our country the distribution per sex of the incidence of Diabetes Mellitus is asymmetrical, but not so that of its complications, a phenomenon that has been discussed by many authors (2, 3, 10). The females predominated in our sample (59.37%). According to Días et al., diabetic females in Cuba outnumber males by approximately 2.5-fold (17.3% vs. 7.7%). Another study on peripheral angiopathies and diabetic foot also found larger numbers of female patients (5).
According to the classification of the disease, 31 out of 32 patients (96.9%) were afflicted with type 2 diabetes (non insulin-dependant). Although the growth in medical knowledge about the etiology of the disease has enlarged the list of diabetes types, for clinical practice (and ignoring cases with a secondary etiology) a two-type classification system continues to be employed. Insulin-dependent Diabetes Mellitus (IDDM) usually appears before 30 years of age, with a relatively sudden onset, tends to ketosis and quickly requires insulin, whereas non-insulin dependant diabetes usually afflicts obese persons older than 40 years, has an insidious onset, and can be controlled in the long-term by diet management, not requiring the use of insulin (19).
The difference between these types does not reside solely in the size of the insular deficit and there is an authentic pathogenetic heterogeneity. Regardless, the appearance of complications is extremely common in both types of diabetes (1, 4).
The basic interest for a clinical classification of the stages of development of diabetic foot ulcers is due, on one side, to the need for adjusting therapeutic protocols to the degree of complication and, on the other, to their predictive value regarding the possibilities of achieving complete cicatrization. In any case, and using a specific and well thought treatment for each type of ulcer, it is still necessary to improve the results regarding the rate of cicatrizations and the number of amputations that have been avoided. The physiopathological classification establishes a distinction between non-ischemic neuropathic ulcer (Grade 3) and ischemic ulcer (Grade 4), with a frequent overlap between both parameters. The most accepted classification is that of Wagner (Meggitt / Wagner) (Table 2), based on 3 parameters (depth, degree of infection and presence of gangrene) (16).
Using this classification, it is found that 10 patients (31.25%) had grade 3 lesions, whereas 22 patients (68.75%) had lesions corresponding to grade 4, since these are the two clinical presentations admitted into the study for the application of Heberprot-P at a dose of 75 µg. This last result has a lot to do with the high ischemic component of the diabetic, which is a predictive factor for an amputation.
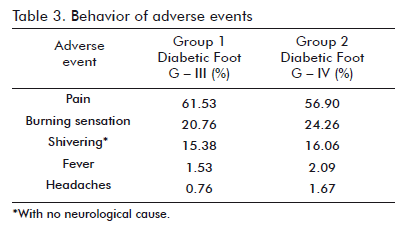
The most frequent adverse events found with Heberprot-P 75 µg were pain, a burning sensation in the site of injection, and shivering (Table 3), as also described during the clinical trials of this drug and for trials involving other topically administered growth factors, such as Beclapernin. During these years, hEGF has also been used to stimulate the cicatrization of different types of internal or peripheral lesions. A critical assessment of these clinical trials and of hEGF treatments examined at different dates indicates that the use of this growth factor in humans is safe, a conclusion supported, in addition, by a large body of data corresponding to the stage of preclinical toxicological testing (4). hEGF is not genotoxic or mutagenic according to internationally validated evaluations, and does not affect cellular epigenetic stability. Its administration might be exceptionally useful in some recalcitrant clinical processes and/ or critical disorders, niches in which its application is appropriately suited to the ethical and therapeutic considerations arising from a risk/benefit analysis for each particular case (4).
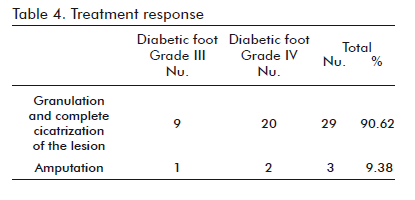
The effect of human Epidermal Growth Factor (hEGF) in the process of cicatrization has been widely studied. The results of this work confirm the biological contribution of hEGF in the field of tissue repair as well as its excellent safety profile, obtaining usable granulation tissue in 90.6% of the patients within an average of 46.6 ± 8.9 days and decreasing the amputation percentage to 9.38% (Table 4). This result is coherent with preceding preclinical assays and clinical trials. In the opinion of the authors, the results concerning the granulation and cicatrization of the ulcers by treatment with Heberprot-P 75 µg were satisfactory in both groups of patients (Figures 1, 2, 3, 4). This investigative report is inspired by the fact that this growth factor has been clinically successful in the repair of peripheral and internal tissues in the face of otherwise unfavorable prognoses for these patients.
CONCLUSIONS
The intralesional administration of Heberprot-P 75 μg solves the contradiction between the loss of EGF and the cicatrization of ulcerative lesions –complicated with infections- on the feet of diabetic patients, allowing granulation, cicatrization, and decreasing the number of amputations among the patients of the “Guillermo Domínguez López” hospital in Puerto Padre, at Las Tunas province.
In all, 49.9% of the patients treated with Heberprot- P 75 µg are older than 70 years. Females were predominant (59.37%), and type 2 diabetes was the most common variant (96.9%). These constitute predictive factors for the appearance of lesions in the feet of the diabetic patient. Of the ulcers, 68.25% were classified as grade 4 (Wagner scale), as expected given the frequent ischemic component of these patients.
Pain, burning sensations and shivering were the most frequent adverse events, of the cases respectively, without the appearance of severe complications resistant to symptomatic treatment.
The efficacy of Heberprot-P 75 µg was proved by a rate of granulation and complete cicatrization of the lesion of 90.62% by the end of the treatment, in a period of 46.5 ± 8.9 days.
The amputation percentage was decreased to 9.38%.
RECOMMENDATIONS
To encourage and alert the basic health service units on the priority that Diabetic Foot constitutes.
To systematize the concept that diabetic foot may be an urgency or a surgical emergency, requiring a rapid decision should amputation be necessary. It should be stressed that it requires a multi-disciplinary approach, which has been proven to be the most effective to treat and prevent the lesions.
To increment the cases to be included in the treatment with Heberprot-P 75 µg so as to achieve an early granulation of the lesions, decreasing the number of amputations due to Diabetic Foot.
To extend this technique to every unit where the optimal conditions for its use exist, as well as to train the personnel selected for its application.
REFERENCES
1. Perich AP, González RM, Valdés E, Arranz MC. Desarrollo de diabetes mellitus en pacientes con tolerancia a la glucosa alterada: Seguimiento de 18 años. Rev Cubana Endocrinol 2002]; 13(2): Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1561-29532002000200002&lng=en (Consultado: 18 de agosto de 2010).
2. Berlanga J, Cibrian D, Guillén I, Freyre F, Alba JS, López-Saura P, et al. Methylglyoxal administration induces diabetes-like microvascular changes and perturbs the healing process of cutaneous wounds. Clin Sci (Lond) 2005;109(1):83-95.
3. Zaulyanov L, Kirsner RS. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging 2007; 2(1):93-8.
4. Berlanga J, Prats P, Remírez D, González R, López-Saura P, Aguiar J, et al. Prophylactic use of epidermal growth factor reduces ischemia/reperfusion intestinal damage. Am J Pathology 2002 Aug; 161(2):373-9.
5. Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg 2006;117(7 Suppl):143S-9S.
6. Mansbridge JN, Liu K, Pinney RE, Patch R, Ratcliffe A, Naughton GK. Growth factors secreted by fibroblasts: role in healing diabetic foot ulcers. Diabetes Obes Metab 1999;1:265-79.
7. Armstrong DG, Lavery LA, Diabetic foot ulcers: prevention, diagnosis and classification. Am Fam Phys 1998;57:1325-32, 1337-8.
8. Boulton AJM, Betts RP, Franks CI, Newrick PG, Ward JD, Duckworth T. Abnormalities of foot pressure in early diabetic neuropathy. Diabet Med 1987;4:225-8.
9. American Diabetes Association. Standard of Medical Care for Patients with diabetes Mellitus. Position statements. Diabetes Care 1998;21:s23-s31.
10. Takehara K. Growth regulation of skin fibroblasts. J Dermatol Sci 2000;24(Suppl 1):S70-7.
11. Mast BA, Schultz GS. Interactions of cytokines, growth factors and proteases in acute and chronic wounds. Wound Repair Regenerat 1996;4(4):411-20.
12. Spencer S. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane database Syst Rev 2005;3:CD002302.
13. Portero-Otín M, Pamplona R, Bellmunt MJ, Ruiz MC, Prat J, Salvayre R, et al. Advanced glycation end product precursors impair epidermal growth factor receptor signaling. Diabetes 2002;51:1535-42.
14. American Diabetes Association: Clinical Practice Recommendations 1997.
15. Foot Care in Patients with Diabetes Mellitus. Diabetes Care 1998;21(Supp 1).
16. Aragón J, Ortiz P, Hernández M. El pie diabético: resultados de nuestra experiencia. Atención Primaria 1998;22:360.
17. Aragón FJ, Hernández MJ, Daniel JM, Ortiz PP. El pie diabético: claves para un diagnóstico y tratamiento adecuados. FOMECO 2000;8:10-25.
18. Armstrong DG, Lavery LA, Harkless LB. A treatment-based classification system for assessment and care of the diabetic feet. J Am Podiatr Med Assoc 1996; 86: 311-316.
19. Marinelo J, Vlanes Jl, Escudero JR, Ibáñez V, Rodríguez J. Tratado de Pie diabético. Jarpyo Editores. España, 2006.
Received in August, 2010.
Accepted for publication in September, 2010.
Wilver Velázquez, Hospital General Docente "Guillermo Domínguez López" Puerto Padre, Las Tunas, Cuba E-mail: fe.tunas@infomed.sld.cu













