My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.27 no.3 La Habana July-Sept. 2010
RESEARCH
Fibrinolysis with recombinant streptokinase affects the prognostic value of cardiac troponin I in acute myocardial infarction: a ten-day follow-up
La fibrinolisis con estreptoquinasa recombinante afecta el valor pronóstico de la troponina I cardiaca en el infarto miocárdico agudo: 10 días de seguimiento evolutivo
Damian Mainet-Gonzalez1, Nolaida Padrón-Brito2, Anselmo Abdo-Cuza3
1 Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 / 158 y 190, Cubanacán, Playa, PO Box 6162, Havana, Cuba
2 Hospital Hermanos Ameijeiras San Lázaro 701 y Padre Varela, Centro Habana, Havana, Cuba
3 Hospital CIMEQ Ave. 11-B / 216 y 11, CP 12100, Siboney, Havana, Cuba
The objective of this multicentre study was to identify the concentrations of cardiac troponin I having a higher probability of cardiovascular and extracardiovascular complications in patients with acute myocardial infarction that were treated with or without fibrinolysis at the intensive care units. These patients were followed for 10 days and divided into two prognostic groups: one without complications (n = 53) and other with clinical complications (n = 28). The plasma extracted from 3 h to 72 h after the onset of symptoms was retrospectively evaluated in an enzyme linked immunoadsorbent assay amplified with streptavidin-biotin for quantifying cardiac troponin I. About 45% of the patients had received recombinant streptokinase for fibrinolysis, from the onset of the symptoms and up to 6 hours. In the fibrinolysis group (n = 32), the concentrations of cardiac troponin I of complicated patients were similar to uncomplicated patients. Cardiac troponin I concentrations in patients without fibrinolysis (n = 49) were higher in complicated than in non-complicated patients (W = 287, p = 0.016). The biological activities of other cardiac biomarkers in complicated patients were as high as in uncomplicated patients treated with or without fibrinolysis. The concentration of cardiac troponin I of 2 ng/mL or higher, was a good predictor of the risk of clinical complications in patients without fibrinolysis (odds ratio: 6.6; 95% confidence interval: 1.5-29.4). In the infarction patients without therapeutic fibrinolysis, cardiac troponin I concentrations could be used to stratify them at the intensive care units and may be evaluated as an indicator of fibrinolysis in other studies.
Key words: cardiac troponin I, fibrinolysis, acute myocardial infarction, short-term prognosis.
RESUMEN
El objetivo de este estudio multicéntrico fue identificar las concentraciones de troponina I cardiaca con mayor probabilidades de complicaciones cardiovasculares y extracardiovasculares en los pacientes con infarto miocárdico agudo que fueron tratados con o sin fibrinolisis en las unidades de cuidados intensivos. Estos pacientes fueron seguidos clínicamente por 10 días y divididos en dos grupos: no complicado (n = 53) y complicado (n = 28). Los plasmas extraídos de los pacientes desde las 3 horas a las 72 horas de inicio de los síntomas fueron retrospectivamente evaluados en un análisis inmunoenzimático en fase sólida amplificado con estreptavidina-biotina para cuantificar troponina I cardiaca. Alrededor del 45% de los pacientes estudiados recibieron fibrinolisis con estreptoquinasa recombinante desde el inicio de los síntomas a 6 horas de evolución. Con la fibrinolisis (n = 32), los pacientes complicados tuvieron las concentraciones de troponina I cardiaca similares a los no complicados. Cuando no se indicó la fibrinolisis (n = 49), los pacientes complicados presentaron mayores concentraciones de troponina I cardiaca que los no complicados (W = 287, p = 0.016). Las actividades biológicas de otros biomarcadores cardiacos fueron similares entre los pacientes complicados y no complicados en el tratamiento con o sin fibrinolisis. La concentración de troponina I cardiaca mayor o igual a 2 ng/ mL fue un buen predictor de riesgos de complicaciones clínicas en los pacientes sin fibrinolisis (razón de productos cruzados: 6.6; intervalo de confianza de 95%: 1.5-29.4). Los pacientes con infarto miocárdico agudo tratados sin fibrinolisis pudieran ser mejor estratificados por riesgos de complicaciones mediante la determinación de las concentraciones de troponina I cardiaca. Este biomarcador pudiera ser evaluado como indicador de fibrinolisis en estudios posteriores.
Palabras clave: troponina I cardiaca, fibrinolisis, infarto miocárdico agudo, a corto plazo pronóstico.
INTRODUCTION
Acute myocardial infarction (AMI) is the first cause of the death in Cuba (1) and several countries of the World (2, 3). AMI is a medical emergency because it has many clinical complications, such as: arrhythmia, cardiogenic shock, transitional cerebral ischemia, chronic renal failure and pulmonary embolism. Any one of these complications can produce death. For this reason, while the diagnosis may be done rapidly, therapeutic decisions -that may be made-could increase the patient´s survival. At the intensive care units, fibrinolysis and percutaneous transluminal coronary angioplasty (PTCA) have decreased the death of these patients. However, about seven out of ten patients with heart pain are admitted in these units (with high technology and qualified personnel) without AMI after a longer period of time, or having AMI without complications due to this cause (3). This affects the quality of medical services because many patients with complications are not able to receive this early attention. On other hand, certain patients could receive less intensive care without affecting the quality of the medical service (2, 4, 5). Cardiac troponin I (cTnI), because of its high cardiospecificity and analytical sensitivity, has been used in the diagnosis of AMI and the evaluation of the prognosis of patients with acute coronary syndrome (ACS; includes acute unstable angina, AMI without ST elevation and AMI with ST elevation) at a short-term, for example: 72 h (6), 24 h and 30 days (7, 8) and 42 days (9, 10). For the short-term prognosis of patients with ACS, the incidence of myocardial reinfarction, arrhythmias, refractory pain, congestive cardiac failure and mortality and an increase of the blood concentrations of cTnI, have been studied. However, no studies have reported the role of cTnI concentrations for predicting other (cardiovascular and extracardiovascular) clinical complications among patients with AMI at the intensive care units after fibrinolysis treatment. It has, however, been demonstrated that the patients with increased concentrations of cTnI in an ACS are more likely to present clinical complications at the short-term follow up (3). Fibrinolysis is a good therapeutic choice in AMI and can save the cardiac muscle when applied as early as possible (11, 12). Due to this treatment, the cTnI concentrations could decrease in the blood. Many patients with AMI reach the intensive care unit after receiving fibrinolysis treatment. In this paper, we report cTnI concentrations in patients with AMI determined in an enzyme linked immunoadsorbent assay (ELISA) after receiving or not receiving fibrinolysis treatment and their prognostic values for detecting any cardiovascular and extracardiovascular complications on the short-term in these patients.
MATERIAL AND METHODS
Groups of patients and biological samples
The evaluation of human beings subjected to research was done following the Helsinki Declaration revised by the 52nd General Meeting of the World Medical Association (Edinburgh, Scotland, October 2000). AMI was diagnosed when the patients after determining two out of three criteria (clinical history, electrocardiogram signs and biochemical criterion) of the World Health Organization for this disorder. The clinical element was the history of chest pain for 20 min or more that did not decrease with nitroglycerine treatment. The electrocardiographic elements showed an elevation of the ST segment higher than 1 mm of limb derivations: DI, DII, DIII, aVL and aVF that registered the same anatomical zone or higher than 2 mm in two or more precordial derivations and left branch block. The patients with these two criteria were considered to have an AMI with ST segment elevation (STEAMI). The biochemical criterion was an increase of biological activities of enzymes in two determinations that were twice as high as their normal reference limits during their diagnostic windows. The concentrations of cTnI were not analyzed for the diagnosis. The patients with chest pain resembling myocardial ischemia and increased cardiac enzyme values, and electrocardiograms without the characteristic ischemic changes were considered infarction patients with non-ST segment elevation (NSTEAMI). The patients with STEAMI received 1 500 000 units of recombinant streptokinase (Heberkinasa, Cuba) by a 60-min intravenous infusion. These patients were classified in two prognostic groups: those with clinical complications (bad prognosis) and another group without complications (good prognosis). The cardiovascular complications were: arrhythmias and ventricular fibrillation, congestive heart failure, cardiogenic shock, extension of myocardial infarction, arterial hypotension or hypertension and cardiac death. The extracardiovascular complications were transitory cerebral ischemia, acute pulmonary edema, bronchopneumonia and acute renal failure.
The plasma samples were obtained at 3 hours of the beginning of chest pain and up to 72 h. The plasma samples of patients with AMI and healthy donors were collected at the coronary or intensive care unit of three hospitals of the City of Havana, Cuba, and the Blood Unit of Marianao during the period of 1999 to 2002. The samples were coded for the study, thereby becoming anonymous samples. The confidentiality of the patient´s personal data was kept throughout the study. The plasma samples were obtained with 4 mmol of ethylen-diamine-tetracetic acid disodium salt dihydrate (EDTA, Fluka, Switzerland) /L of blood and 2 IU (international units) of heparin (Imefa, City of Havana, Cuba) / mL of blood as anticoagulants. The samples with haemolysis were discarded. All samples after thawing were homogenized and centrifuged at 4 000 g to eliminate the fibrin clots.
Data collection and follow-up
We collected the following variables from the patient files: age, gender, race or skin color, weight, history of hypertension, diabetes mellitus, hyperlipidoemia, smoking, previous myocardial infarction, muscular trauma, chronic renal failure, chronic muscular disease, hepatic disease and chest contusion. The general physicians obtained the follow-up information for 10 days at the intensive or coronary care unit.
ELISA for quantifying the concentration of cTnI
This method has been described before (13) and the monoclonal antibodies (Mab) used, recognize the stable part of the cTnI molecule as free and forming a ternary complex (cardiac troponin C-cardiac troponin T-cTnI) or a natural cardiac troponin complex. The CBTnI16 and CBTnI11 Mab (Immunodiagnostic and Genomic Division of CIGB, Cuba) recognize the epitopes of cTnI: 89-FAELQD-94 and 26-YRAYAT-31, respectively. The 19C7 Mab (Hytest, Turku, Finland) binds the epitope 41- SASRKLQLK- 49. Briefly, the microtitter plates (Maxisorp, Roskilde, Denmark) were coated with 15 µg/mL of CBTnI 16 Mab diluted in 50 mmol/L carbonate bicarbonate buffer pH 9.6 during 16 h. After the free sites of plates were blocked with phosphate buffer of saline (PBS), 2% (w/v) skim milk (Oxoid, United Kingdom) and tween-20 0.05% (v/v) placed at 37 ºC for 1 h applying 100 µL/well. The plasma samples and biotinylated 19C7 and CBTnI11 Mab were separately diluted in the blocking solution. The samples and the biotinylated Mab solution were added to the plate at the same step and were incubated together for 15 min at 37 ºC under 650 rpm agitation in the Titramax 100 machine (Heldolph Instruments, Germany). The plasma had a final dilution factor of 1/4 to 1/16. The standard curve was prepared with troponin I in a ternary complex that was diluted in blocking solution with 25% (v/v) plasma that was formed by a pool of different donors. The streptavidine conjugated to peroxidase (Amersham Pharmacia, Uppsala, Sweden) was diluted 1/1000 in the PBS tween-20 0.05% and was incubated for 10 min at 37 ºC under agitation. After that, the orthophenylenediamine dihydrochloride in the substrate buffer was added to the plates. The enzymatic reaction was stopped with 50 µL/well of 2.5 mol/L sulphuric acid and the plates were read in the PR 521 plate reader (Tecnosuma International, City of Havana, Cuba) at 492 nm. The samples were considered positives where the absorbances of wells were higher than the mean absorbance plus 2 standard deviations of those wells with a blocking solution in 25% plasma. The calibrator was the natural cardiac troponin complex (Hytest, Turku, Finland). That reagent was diluted in the human serum pool of healthy donors at 1 mg/L and filtered in the cellulose acetate with a 0.45 µm cut-off (Sartorious, Germany). The human cTnI represents 30% of the molecular weight of the cardiac troponin complex. The concentration of cTnI in the cardiac troponin complex solution was 300 ng/mL. The detection limit of this assay was 0.1 ng/mL of cTnI and the standard curve had a range of 0.2 to 3.5.
Other biological assays
The quantification of the biological activities of cardiac biomarkers: creatine quinase (CK, Slave diagnostic, Siena) and isoform MB of CK (CKMB), aspartate aminotransferase and the L-lactate dehydrogenase (Roche Boehringer, Mannheim, Germany) were done following the manufacturer´s instructions. The upper reference limits were 195 IU/L for CK, 18 IU/L for CKMB, 37 IU/L for aspartate aminotransferase and 450 IU/L for lactate dehydrogenase.
Statistical analysis
The odds ratio (OR) and its 95% confidence interval (95% CI) and chi-square and its associated probability were calculated for contingency tables in Statgraphic plus 5.1 for Windows (Statistical Graphics Corp, USA). The receiver operating characteristic (ROC) curve and the area under curve (AUC) using Hanley and McNeil formulation (14) were done with Epidat software (Galicia, Spain). The parametric (t-student) and non-parametric (Mann Whitney) hypothesis tests were considered significant when the two-tailed probability was lower than 0.05. All results for continuous variables are expressed as means +/- standard deviations and categorical data as percentage. The positive (LR+) and negative (LR-) likelihood ratios were calculated using the formula: LR+ = sensitivity/ (1-specifi city) and LR- = (1-sensitivity)/specificity (14). The Microsoft Excel software (Window, USA) was used for organizing the data.
RESULTS AND DISCUSSION
First, some considerations about the detection limit and the cTnI standard used in the immunoassay and the determination time of cTnI from the onset of chest pain to 72 h will be discussed according to the purpose of this study. At present, there are many enzyme immunoassays with a detection limit that is lower than the one that was used in this study (0.1 ng/mL). For example, the detection limits of 0.034 ng/mL (15) and 0.015 ng/mL (16) have been reported in different immunoassays. In spite of that, the cut-off value of AMI is considered to be > 0.09 ng/mL (16). The patients treated here had AMI. For this reason, it was unnecessary to have an immunoassay with very high analytical sensitivity. The calibrator used here has been suggested due to the stability of cTnI in the ternary complex. Furthermore, it enables a decrease of the variation of different assays of cTnI; although it is still unknown how the cTnI is released from the dead cardiac cell into the plasma (17, 18). There are no differences for identifying high risk patients with ACS when the cTnI concentrations are collected in the first determinations (OR: 4.66; 95% IC: 3.12-6.97) and those based on the concentrations of the cTnI peak (OR: 5.04; 95% IC: 3.3-7.71) (7). Ottani et al. (7) suggests taking at least 2 samples of blood in the first 24 h to study the cTnI concentrations in the prognosis of these patients. In the present study, the concentrations of cTnI were obtained in the first 72 h after the onset of symptoms and some patients had more than one determination. The objective of this study was to find the clinical significance of concentrations of cTnI higher than 0.1 ng/mL and to see if it were possible to prove the stratification concept of patients with AMI accepted at intensive care units through short-term prognosis, and to find a value of this biomarker for this assumption.
This study included 81 patients with AMI and 28 (35%) of them presenting clinical complications. Some patients (n = 10, 12%) had two or more complications and the total number of complications was 44. The relative frequencies of cardiovascular complications were: 45% for arrhythmias and ventricular fibrillation (absolute frequency = 20), 30% for congestive heart failure (absolute frequency = 12), 11% for extension of infarct, arterial hypotension or hypertension, cardiogenic shock and cardiac death (absolute frequency = 6). The relative frequencies of extracardiovascular complications were: 2% for transitory cerebral ischemia, (absolute frequency = 1), 7% for acute pulmonary edema and bronchopneumonia (absolute frequency = 3), 5% for acute kidney failure (absolute frequency = 2). Figure 1 shows the relative frequency of clinical complications in patients with AMI treated with or without the fibrinolysis agent. The relative frequency of cardiovascular complications was higher than those of extracardiovascular complications (calculated Z = 3.84, p = 0.0001). The frequencies of cardiovascular and extracardiovascular complications of patients with the fibrinolysis treatment were similar to the untreated patients (Figure 1). The main personal pathological backgrounds of these patients were arterial hypertension, previous AMI, and peripheral arteriopathy (absolute frequency = 25 for a relative frequency: 57%) (Table 1).
The relative frequency of patients with ST electrocardiographic segment elevation (t = 2.2, p = 0.028) and brown skin (z = -2.38, p = 0.017) were higher in patients with complications than in uncomplicated patients (Table 1). The onset of fibrinolysis of the infarction patients with complications was faster than with uncomplicated infarction patients. The rest of the clinical variables did not show significant differences between complicated and uncomplicated patients with AMI. The elevation of the ST electrocardiographic segment (84.6% non-survivals, n = 47 versus 14.6% survivals, n = 11 p < 0.000 1) has been reported to be statistically different in the baseline characteristic between the group of patients with or without the risk of death and reinfarction when they were admitted with the assumption of ACS (8). In that study, the cTnI concentrations were higher in the patients that did not survive than patients that did survive. Nevertheless, the concentrations of cTnI were found to have no significant differences between infarction patients with complications and those without complications in our study. In spite of that, mean concentrations of cTnI in complicated patients were twice as high as those of uncomplicated patients. Due to the fact that the group of patients included were only those with AMI and about 40 to 50% of them had received the fibrinolysis treatment with recombinant streptokinase at the intensive care rooms, we believe that the fibrinolysis could have a protective effect on the myocardium and could decrease the cardiac damage and the concentrations of cTnI in those patients, above all, if this treatment was carried out as soon as possible. In our study, the mean starting time of fibrinolysis varied between 2 h and 4 h after the onset of symptoms of STEAMI (Table 1). That period in patients with a bad prognosis was shorter than in the patients with a good prognosis (W = 19.5, p = 0.015). This element can lead to a decrease of the area of damaged tissue and the post-infarction clinical complications in these patients (1). For these reasons, this biomarker was studied for prognosis in the group of patients with AMI receiving or not receiving the fibrinolysis treatment. The infarction patients having fibrinolysis had cTnI concentrations that were not significantly different from the patients that were not treated with fibrinolysis, compared in both the complicated and uncomplicated groups.
The concentrations of cTnI in patients treated without fibrinolysis were significantly (W = 287, p = 0.016) higher in the group with a bad prognosis than in the group with a good prognosis (Figure 2). However, in patients treated with fibrinolysis the concentrations of cTnI of complicated patients were as high as in uncomplicated patients. The clinical baseline characteristics of the two groups of patients in the first comparison did not show significant differences. In the second comparison, the clinical baseline characteristics of the two groups of patients only showed statistically significant differences in the relative frequency of patients with brown skin. This characteristic in complicated patients was more frequent than in uncomplicated patients (z = - 2.01, p = 0.044) in a small size sample (n = 10). For these reasons, the concentrations of cTnI as a prognostic factor for AMI were studied in patients without fibrinolysis.
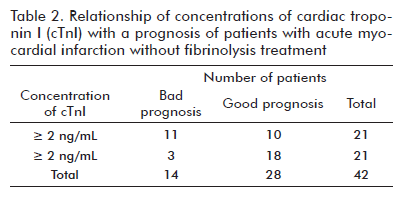
The concentrations of cTnI in the ROC curve identified clinical complications in the infarction patients without fibrinolysis (AUC: 0.73, 95% CI: 0.56 a 0.90). This result suggests that the concentrations of cTnI discriminated both groups of (complicated and uncomplicated) patients. The Szymanski´s group (8) reported AUC: 0.76 (95% CI: 0.7-0.81) for cTnI concentration and 0.78 (95% CI: 0.72-0.83) for CKMB mass for only identifying death in patients with suspected ACS at their admission at the emergency ward, at which at a time the patients had not received fibrinolysis treatment. In that study, the different values of LR+ and LR- are not described. In the present study, the LR + and LR - for different cut-off values were less than 10 and higher than 0.1, respectively. The best result was for 2 ng/mL of cTnI that presented LR+ of 2 and LR- of 0.3. These likelihood ratios generate small changes from pre-test to post-test probabilities, and the diagnostic power of cTnI as a biomarker of complications in patients with AMI is low (14). For this reason, the concentrations of cTnI cannot be chosen as a biomarker of complications in these infarct ion patients without fibrinolysis treatment. Nevertheless, this study enabled the analysis of 2 ng/mL of cTnI as a risk factor (Table 2). That concentration of cTnI had a strong association with the type of prognosis of the infarction patient without fibrinolysis (corrected chi square: 5.2; degrees of freedom: 1; p = 0.02). These patients had a higher probability of cardiovascular and extracardiovascular complications in a short-term follow-up when the concentrations of cTnI were equal or higher than 2 ng/mL (OR: 6.6; 95% CI: 1.5-29.4). This absolute value is similar to the results of Stephen Hill (6) when analyzing all patients with ACS and studying only cardiac complications in 72 h of follow-up. The absolute value between cTnI assays should not be compared because there is no commutability in the determination of this biomarker for different assays (there is no universal standard material for cTnI determinations and there is a difference in antibody epitope recognition between assays). However, the clinical interpretation for each study with distinct cTnI assays for diagnosing AMI is available. Both studies confirm the existence of a value of cTnI concentration as the predictor of clinical complications at very short term. Antman EM et al. (9) found that cTnI concentrations of over 0.4 ng/mL increased the risk of reinfarction and mortality in 42 days of evolution when they studied about 1000 patients with acute unstable angina and non-Q AMI. In that paper, the patients with fibrinolysis administered within 72 h or PTCA performed in the previous 6 months were excluded. In the present study, the AMI patients treated with fibrinolysis or PTCA were not excluded and renal, cerebral and pulmonary complications were also studied during ten days of the follow-up. The group of patients studied here was more homogeneous than in the above study.
This study presents certain limitations that allow us to make certain recommendations. The sample size should be larger in different periods (0-12 h, 13-48 h and 49-72 h) since the onset of the symptoms. The design of this study should be prospective, which is a more real condition than a retrospective design. If a cut-off value with LR + ³ 10 and LR- £ 0.1 (that does not depend on the prevalence) is found, the cTnI concentration could be chosen as a biomarker of complications in patients with AMI admitted at coronary or intensive care units. This concentration of cTnI could be used at the used at the ward testing the cut-off of complications in patients with AMI. Perhaps, this concentration could be studied as a criterion of fibrinolysis or another therapy in these patients for decreasing the risks of complication. Gunn et al. (19) have demonstrated the successful use of fibrinolysis in patients without electrocardiographic criteria, but with clinical (chest pain, smoking, hypertension, hyperlipidaemia, family pathological history of atherosclerosis, etc) and biochemical (the increasing of CKMB mass determinations) criteria of AMI. However, they suggest that this approach be demonstrated in a sufficiently large group of patients and analyze the cost-effective ratio in practice when using the streptokinase therapy for saving the lives in these patients. Certain authors (20, 21) have also stated that the prediction of thrombolytic therapy using a biomarker before 12 h after the beginning of symptoms will be beneficial. The biomarkers studied for this purpose are the concentrations of CKMB mass (19, 20), CK activity (21), cardiac troponin T (20, 22) and the combination of CKMB mass, cardiac Troponin T and myosin light chain (23). However, there are no results demonstrating a cardiac biomarker or a group of them to be an indicator of fibrinolysis as suggested by Gunn et al. That study could be repeated in patients with AMI using cTnI, and also CKMB concentrations in patients with cardiac damage without electrocardiographic criteria. Recently, the copeptin has been reported as a new rapid biomarker of AMI (24), we believe that it could be studied together with cardiac troponins in the stratification and therapeutic option of fibrinolysis of these patients.
In a previous paper (13), the authors found a patient with STEAMI at the diaphragmatic region that had negative concentrations or activities of cTnI and other cardiac biomarkers at 72 h. This patient had received fibrinolysis with recombinant streptokinase at 90 min after the onset of the chest pain. We hypothesize that the rapid treatment with fibrinolysis could have saved the myocardium tissue and decreased the time of hypoxia and may have affected the AMI diagnosis of cardiac biomarkers in this patient. Now, in this paper we demonstrated that the fibrinolysis with recombinant streptokinase within six hours after the onset of the symptoms changes the prognosis value of the cTnI in patients with acute myocardial infarction using a 10 day follow-up. This present study suggests that a group of patients with AMI symptoms that did not show characteristic ischemic changes in their electrocardiograms, but had concentrations of cTnI of 2 ng/mL or higher, should be admitted at the intensive care units more rapidly than other patients with lower concentrations of cTnI. This decision can decrease hospital cost without affecting the quality of medical services. This care is better adjusted to the pathological state of these infarction patients. Furthermore, the present study suggests that these patients should receive fibrinolysis as another therapeutic option that may bring about better prognosis. The number of patients with AMI that receive fibrinolysis treatment should be increased with this new laboratory criterion when studies are made on how this criterion could affect the benefit/risk ratio of fibrinolysis with recombinant streptokinase in a new group of patients.
CONCLUSIONS
In the group of patients with AMI accepted in the intensive care ward: the elevation of the ST electrocardiographic segment, the time between onset of chest pain and the start of the fibrinolysis treatment and the frequency in persons with brown skin were found to be different in the baseline characteristics between complicated and uncomplicated patients with AMI. The concentrations of cTnI did not give prognostic information in patients with AMI that had received fibrinolysis at the start of the chest pain and with 6 h of evolution. However, the concentration of cTnI was a good predictor of clinical complications on the short term in patients with AMI admitted at the intensive care units and not receiving fibrinolysis treatment. Thus, it has been demonstrated for first time in this paper that therapeutic fibrinolysis affects the prognostic value of cTnI concentrations in patients with AMI: These patients without fibrinolysis and with concentrations of cardiac troponin ³ 2 ng/mL should be assisted more urgently in the intensive care units than the infarction patients with lower concentrations of this biomarker.
ACKNOWLEDGMENTS
The authors wish to thank the doctors and technicians of the Hermanos Ameijeiras and CIMEQ Hospitals and the Cardiology and Cardio-vascular Surgery Institute for collecting the biological samples and the clinical data at the intensive care or coronary care units. The authors are very grateful to Carmen Valenzuela for offering us the Epidat software and her statistical suggestions
REFERENCES
1. Mortalidad del Anuario Estadístico 2007. Estadísticas de Salud en Cuba. Available from URL:http://www.infomed.sld.cu/servicios/estadisticas/ (august 3, 2009).
2. Eisenman A. Troponin assays for the diagnosis of myocardial infarction and acute coronary syndrome: where do we stand? Expert Rev Cardiovasc Ther 2006;4:1-6.
3. Ekelund U, Forberg JL. New methods for improved evaluation of patients with suspected acute coronary syndrome in the emergency department. Postgrad Med J 2008;84:83-6.
4. Piñón-Pérez J, Cabrera-Cabrera R, Pita- Serantes C. Infarto miocárdico agudo ¿un diagnóstico fácil de realizar? Rev Cubana Med 2000;39:96-100.
5. Hamiltona AJ, Swalesb LA, Neilla J, Murphya JC, Darragha KM, Rockeb LG, et al. Risk stratification of chest pain patients in the emergency department by a nurse utilizing a point-of-care protocol. Eur J Emerg Med 2008;15:9-15.
6. Hill SA, Devereaux PJ, Griffith L, Opie J, McQueen MJ, Panju A, et al. Can troponin I measurement predict short-term serious cardiac outcomes in patients presenting to the emergency department with possible acute coronary syndrome? Can J Emerg Med 2004;6:22-30.
7. Ottani F, Galvani M, Nicolini FA, Ferrini D, Pozzati A, Di Pasquale G, et al. Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 2000;140:917-27.
8. Szymanski FM, Grabowski M, Filipiak KJ, Karpinski G, Hrynkiewicz A, Stolarz P, et al. Prognostic implications of myocardial necrosis triad markers´ concentration measured at admission in patients with suspected acute coronary syndrome. Am J Emerg Med 2007;25:65-8.
9. Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996;335:1342-9.
10. Kalra N, Sattur S, Sorrell VL. Prognostic value of megatroponinemia after myocardial infarction. Am J Med 2009;122:392-4.
11. Gupta A, Sabatine MS, O´Gara PT, Lilly LS. Acute Coronary Syndromes. In: Lilly LS, editor. Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty. Third edition. Philadelphia: Lippincott Williams & Wilkins; 2003. p.157-84.
12. Bazart-Padrón P, Correa-Torres M, Ramos-Gutiérrez LB, Lóriga-García O. Aplicación de estreptoquinasa recombinante en el infarto miocárdico agudo. Rev Cienc Méd Pinar del Río 2003; 7(2).
13. Mainet-Gonzalez D, Sorell-Gómez L, Pichardo-Díaz D, Reyes-Acosta O, Torres-Cabrera MB, Abdo-Cuza A, et al. Evaluation of a rapid immunoassay for the quantification of cardiac troponin I in the diagnosis of acute myocardial infarction (Spanish). Químa Clín 2003;22:419-30.
14. Henderson AR. Assessment of clinical enzyme methodology: a probabilistic approach. Quim Acta 1997;257:25-40.
15. Apple FS, Pearce LA, Smith SW, Kaczmarek JM, Murakami MM. Role of monitoring changes in sensitive cardiac troponin I assay results for early diagnosis of myocardial infarction and prediction of risk of adverse events. Clin Chem 2009; 55:930-7.
16. Di Serio F, Lovero R, Leone M, De Sario R, Ruggieri V, Varraso L, et al. Integration between the Tele-Cardiology Unit and the central laboratory: methodological and clinical evaluation of point of-care testing cardiac marker in the ambulance. Clin Chem Lab Med 2006;44:768-73.
17. Bunk DM. Reference Materials and Reference Measurement Procedures: An Overview from a National Metrology Institute. Clin Biochem Rev 2007;28:131-7.
18. International Federation of Clinical Chemistry (IFCC), Committee on Standardization of markers of Cardiac Damage (CSMCD) members and National Academy of Clinical Biochemistry (NACB) Committee members. NACB and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: Analytical Issues for Biochemical Markers of Acute Coronary Syndromes. Circulation 2007;115:e352-5.
19. Gunn IR, Noreen SM, Parnham AJ, Caslake CE, Mathews DM and O´Brien IAD. Emergency serum creatine kinase MB isoenzyme concentration in patients with suspected acute myocardial infarction. Health Bulletin 1993;51(3):166-76.
20. Keffer JH. Myocardial markers of injury: Evolution and Insights. Am J Clin Pathol 1996;105:305-20.
21. Downie AC, Frost PG, Fielden P, Joshi D, Dancy CM. Bedside measurement of creatine kinase to guide thrombolysis on the coronary care unit. Lancet 1993;341: 452-4.
22. Ravkilde J, Nissen H, Hørder M, Thygesen K. Independent prognostic value of serum creatine kinase isoenzyme MB mass, cardiac troponin T and myosin light chain levels in suspected acute myocardial infarction. Analysis of 28 months of followup in 196 patients. J Am Coll Cardiol 1995;25:574-81.
23. Ohman EM, Armstrong PW, Christenson RH, Granger CB, Katus HA, Hamm CW, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med 1996;335:1333-41.
24. Keller T, Tzikas S, Zeller T, Czyz E, Lillpopp L, Ojeda FM, et al. Copeptin improves early diagnosis of acute myocardial infarction. J Am Coll Cardiol 2010;55: 2096-106.
Received in December, 2009.
Accepted for publication in July, 2010.
Damian Mainet-Gonzalez, Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 / 158 y 190, Cubanacán, Playa, PO Box 6162, Havana, Cuba. E-mail: damian.mainet@cigb.edu.cu













