Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.27 n.4 La Habana oct.-dic. 2010
RESEARCH
Factors influencing adsorption of Dermatophagoides siboney allergen extract into aluminum adjuvants
Factores que influyen en la adsorción del extracto alergénico del Dermatophagoides siboney en adyuvantes que contienen aluminio
Cam Anh Nguyen1, Yarima Alonso2, Yunia Oliva2, Alexis Labrada2, Orestes Mayo3
1 Division of Medical Biotechnology, Biotechnology Centre of Ho Chi Minh City, Vietnam
2 Allergens Department, National Center of Bioproducts, Havana, Cuba
3 Development Department, National Center of Bioproducts, Havana, Cuba
ABSTRACT
Allergen-specific immunotherapy consists in periodic administration of allergen vaccines, particularly from House Dust Mites (HDM), for desensitization and amelioration of allergic symptoms. The mite Dermatophagoides siboney has been commonly found in house dust in the Caribbean and it is associated to allergic asthma. In order to obtain a HDM vaccine containing aluminum adjuvant that can satisfy the requirements of consistency and immunogenicity, a lyophilized allergen extract of D. siboney was adsorbed into aluminum hydroxide (AH) and aluminum phosphate (AP) gels, aiming to establish the parameters that determine the highest adsorption capacity. Allergen adsorption was measured by total protein assays and by Der s 1 allergen-specific MAb-ELISA. Immunological potency was assessed in BALB/c mice. The results showed that AH had better adsorption capacity when compared to AP. The best adsorption conditions using AH were: 0.9% NaCl at pH 8 in 30 min. Sodium phosphate - buffered solution had negative effect to the allergen adsorption into AH, when used during the process or added later. The within-batch consistency of the adsorption process in the absence of buffer was demonstrated as well as the immunogenicity of this formulation, regarding induction of allergen-specific IgG antibodies.
Keywords: Vaccine formulation, allergens, adsorption process, aluminum adjuvants, phosphate buffer.
RESUMEN
La inmunoterapia alergeno específica consiste en la administración periódica de vacunas alergénicas, particularmente del Ácaro del Polvo Casero (HDM), para la insensibilización y disminución de los síntomas alérgicos. El ácaro Dermatophagoides siboney se encuentra comúnmente en el polvo de las casas en el Caribe y se asocia con el asma alérgica. El extracto alergénico liofilizado de D. siboney se adsorbió en geles de hidróxido de aluminio (AH) y fosfato de aluminio (AP), con el fin de obtener una vacuna de HDM conteniendo aluminio que satisfaga los requerimientos de consistencia e inmunogenicidad y así establecer los parámetros que determinan una mayor capacidad de adsorción. La adsorción del alergeno se midió por el ensayo de proteínas totales y por un MAb-ELISA alergeno específico para Der s 1. La potencia inmunológica se evaluó en ratones BALB/c. Los resultados mostraron que el AH tiene una mayor capacidad de adsorción comparada con la del AP. Las mejores condiciones de adsorción usando AH fueron: 0.9% NaCl a pH 8 en 30 minutos. La solución tampón de fosfato de sodio tuvo un efecto negativo en la adsorción en AH, tanto añadida durante el proceso o al final del mismo. La consistencia entre lotes del proceso de adsorción en ausencia del tampón fue demostrada, así como la inmunogenicidad de esta formulación referida a la inducción de anticuerpos IgG alergeno específico.
Palabras clave: formulación de vacunas, alergenos, proceso de absorción, adyuvantes de aluminio, tampón fosfater.
INTRODUCTION
House dust mites, including Dermatophagoides siboney have been causing serious allergic diseases worldwide, such as dermatitis, eczema and asthma. Allergen- specific immunotherapy (SIT) is the only etiologic treatment of allergic diseases available worldwide and capable of interfering the disease progress. (1) Allergen vaccines prepared from native allergen proteins, including House Dust Mites (HDM), in aqueous or adsorbed form are preferentially used for SIT.
Alum adjuvants, including Alum Phosphate (AP) and Alum Hydroxide (AH), are known to raise and modulate the immune response and are used for many human vaccines including allergen vaccines. Achieving a high degree of adsorption is known to be relevant for the adjuvant effect or antigen uptake, and therefore for vaccine efficacy (2). Generally, the adsorption process is carried out by mixing the adjuvant and the antigen, at optimal pH, temperature, solution concentration, and appropriate stirring velocity in a defined time. There are three major interactions in the adsorption process of the antigens into alum adjuvants: electrostatic attraction, hydrophobic interaction and ligand exchange. Electrostatic attraction is the most important and occurs when the adjuvant and the antigen have opposite charges. It depends heavily on the isoelectric point (pI) of the antigen and the point of zero charge (PZC) of the adjuvant. Then, the adsorption process is best accomplished in the pH interval between these two values. AH has been identified as a poorly crystalline aluminum oxyhydroxide (3) with a PZC of 11, which favors adsorption of negatively charged proteins at neutral pH. In contrast, AP has been classified as an amorphous aluminum hydroxyphosphate with a PZC of 5 favoring adsorption of positively charged proteins. The allergen extract of D. siboney contains predominantly the major allergen protein Der s 1 with a pI in the range of 5.9 - 6.8. (4). Hence, theoretically, it would be readily adsorbed into AH, taking advantage of their opposite charges in contrast to AP. Figure 1 depicts the situation described before. Nevertheless, previous experience has shown that the adsorption process of Der s 1 into AH can be inconsistent and suboptimal. In fact, electrostatic attraction is only one among the three most important interactions of the adsorption process. Hydrophobic interactions and specially ligand exchange could also play a role in this process. In addition, salt ions, and particularly buffers containing phosphates, commonly used in vaccine formulation, could affect the adsorption capacity of alum adjuvants (5).
Hence, the aim of this work was to select the most suitable adjuvant from AH and AP in order to achieve consistently the highest adsorption values with a desired immunogenic effect and to determine the optimal conditions for the adsorption process.
MATERIALS AND METHODS
Vaccine formulation
A lyophilized allergen extract of HDM D. siboney (VALERGEN-DS, 100 000 BU) manufactured by BIOCEN, Cuba, was used as the antigen. Der s 1 content as measured by MAb-ELISA, was used as a concentration marker. AP 2% (Adjuphos) and AH 2% (Alhydrogel) were obtained from Brenntag Biosector (Frederiksuund, Denmark). The vaccine was formulated at a final Der s 1 concentration of 10 mg/mL, 1 mg/mL of either AP or AH and 0.05% of thiomersal. Different buffers were used depending on the experimental design: Tris-(hydroxymethyl)-aminomethan 25 mmol/L at pH 6.8 or 8.8; sodium phosphate-buffered saline (sPBS) pH 7.2 which includes di-sodium hydrogen phosphate 8.4 mmol/L, sodium dihydrogen orthophosphate 4 mmol/L and sodium chloride 136 mmol/L. For the last experiment design the NaCl 0.9% unbuffered solution was used at adjusted pH values of 7.0 or 8.0, using NaOH or HCl. Water for injection was used in all the experiments.
Analytical methods
The adsorption of the antigen onto the adjuvant gel was assessed by measuring protein or allergen content in the vaccine´s supernatant, i.e. after a brief centrifugation separating the gel from the buffer solution.
Protein content was assayed using Comassie-blue staining method of Bradford (6) in a microplate format, with a Bovine Serum Albumin (BSA) standard curve, ranging from 0 to 200 mg/mL. The absorbance at 620 nm was measured using a Microplate reader (Multiskan MS). Samples of vaccine supernatants were applied diluted in this assay.
The major allergen Der s 1 content was measured by a sandwich MAb-ELISA as described by Serwer et al.(7). Briefly, Nunc Maxisorp microplates were coated with 5F7H8F2 capture antibody (BIOCEN, Cuba). After blocking with 1% BSA and washing out non-specific bound proteins using PBS-Tween 20, the vaccine´s supernatant samples were applied at dilutions ranging from 10 to 320. After washing, bound Der s 1 allergen was detected using the biotin-labeled 4C1 MAb (BIOCEN, Cuba) and streptavidine peroxydase (SIGMA, USA) dilution 1:1000. An in-house standard curve of Der s 1 was used ranging from 25 ng/mL. Reaction was developed using 3,3´,5,5´-tetramethylbenzidin (TMB) and H2O2 as substrate in citrate phosphate solution, and stopped adding sulfuric acid 2.5 mol/L. Color intensity was assessed by measuring the absorbance at 450 nm using a microplate reader (Multiskan MS).
Murine allergen-specific IgG antibodies were measured by an indirect ELISA. Nunc Maxisorp microplates were coated with 1 mg/well of the lyophilized D. siboney allergen extract (BIOCEN) in sodium carbonate - bicarbonate at pH 9.6. After incubation overnight at 4 ºC and washing out with PBS-T, blocking was performed using BSA 1% for 1 hour at 37 ºC. After washing with PBS-T, 100 mL of each mouse sera were added into each well, diluted in 1:100 in BSA 1%, and incubated for 2 hours at 37 ºC with slow agitation. Following the washing step, 100 mL of the anti-mouse-IgG biotin conjugate (SIGMA, USA) were applied into each well at a 1:1000 dilution, and incubated for 1 hour at 37 ºC with slow agitation. Finally, after washing, 100 mL of streptavidine peroxydase (SIGMA, USA, 1:5 000 dilution) was added and incubated for 30 minutes at room temperature. The reaction was developed adding a tablet of substrate mixture (Urea/H2O2 and ortophenilendiamine, OPD, SIGMA, USA) and stopped with sulfuric acid 2.5 mol/L. Color intensity was assessed by measuring the absorbance at 450 nm using a microplate reader (Multiskan MS). The results are expressed in optical density (OD) units.
Immunogenicity study in a murine model
Immunogenicity of vaccine formulation variants was tested by injecting two doses (10 mg Der s 1) into BALB/c mice (CENPALAB, Cuba) with a 10 dayinterval by subcutaneous route. Mice were randomly allocated in 4 groups with 6 mice in each group (3 males and 3 females). Groups A, B and C were administered with three consecutive pilot batches of the vaccine variant formulated with AH in saline solution (without buffer). Group D was immunized with the negative control (AH adjuvant in saline solution). Blood was extracted at days 0 and 17 and mice were sacrificed at day 17. Serum allergen specific antibodies were evaluated by ELISA as described before.
Statistical design and analysis
The design of the second adsorption experiment and all the analyses were performed using Statgraphic Centurion XV.II software (StatPoint, Inc):
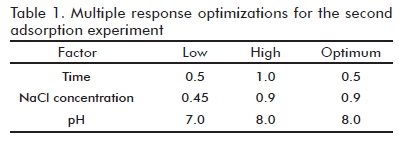
A full 23 factorial design (screening design) consisted in all combinations of 2 levels (low and high) of the 3 experimental factors: pH, time and NaCl concentration in unbuffered solution. All these parameters were randomly ordered with one replicate to form the 16 runs experiment design. Experimental results were later processed to determine each optimal response. Finally, the multiple response optimization were used to find out the best formulation conditions.
Comparing groups in the immunization experiment was performed by using two - way analysis of variance (ANOVA) with multiple sample comparisons. Multiple range test was used to arrange the samples into homogenous groups with no statistical difference between their means.
RESULTS
A preliminary experiment was carried out to choose the best adjuvant between AP and AH for the adsorption process and to define the best formulation solution selecting buffers among sPBS, Tris and unbuffered NaCl . As shown in figure 2, the Der s 1 adsorption values of AH for all buffer solutions were higher than those of AP. Thus, AH was chosen as the best option for next experiments. Among the results of AH, the adsorption process using Tris (pH 8.8) and NaCl rendered the highest adsorption percentages: 96.55% and 95.63%, respectively; meanwhile, the lowest value was shown with sPBS. Although Tris buffer showed the highest adsorption value, NaCl solution was chosen for further experiments due to its well-established compatibility for the injectable route of administration, from the lack of toxicity point of view.
Since AH was the best adsorptive adjuvant, a second adsorption experiment was performed to define the optimal conditions for the adsorption process using AH. The screening experimental design was carried out with 3 factors (adsorption time, NaCl concentration and pH) at 2 levels (low and high) with one replicate. NaCl solution was utilized at eight different conditions: concentrations 0.45% and 0.9%, adjusted at pH 7 or 8, and time intervals of 0.5 and 1 hour, in order to determine the effect of these variables on the adsorption process. No changes of the pH values were detected during the formulation process.
Main Effect plots of the three variables, regarding their influence on adsorption of the Der s 1 allergen and total protein are shown in figure 3. Regarding Der s 1 adsorption (the main quality parameter) the variable with the greater (significant) effect was the NaCl concentration (Figure 3B): increasing this concentration from 0.45 to 0.9% significantly decreased the amount of target antigen in the supernatant, i.e. increased the adsorption effect (Figure 3A). The influence of the other variables (time and pH) was not significant, (p > 0.05). In contrast, saline concentration was not significant regarding adsorption of total proteins, as measured by the Bradford technique. Unexpectedly, increasing time from 0.5 to 1 hour decreased protein adsorption values. On the other hand, increasing pH from 7.0 to 8.0 significantly improved total protein adsorption (Figure 3C). The interaction between time and saline concentration was significant according to Pareto analysis (Figure 3D). Taking into account these apparently contradictory results a multiple response optimization was performed (The results are shown in table 1). According to this approach, the optimal variant chosen for further experiments was a saline concentration of 0.9% (corresponding to physiological level), 0.5 hour of adsorption time and a pH value of 8.0.
Using fixed optimized conditions, a third experiment was performed in order to check if the addition of buffer (sPBS) would, in fact, affect the adsorption process, as seen in the first preliminary experiment. AH adjuvant was used as before, to adsorb the allergen into NaCl formulation solution in presence or absence of sPBS buffer.
The results of this experiment confirmed the deleterious effect of phosphate buffer on Der s 1 adsorption. The highest adsorption values (more than 99%) were obtained by the variants without sPBS (runs 1 and 2, Figure 4). Adding sPBS either during the adsorption process (run 3) or at the end of this process (runs 1B and 2B), resulted both in lower and insufficient adsorption values, ranging from 63% to 65%.
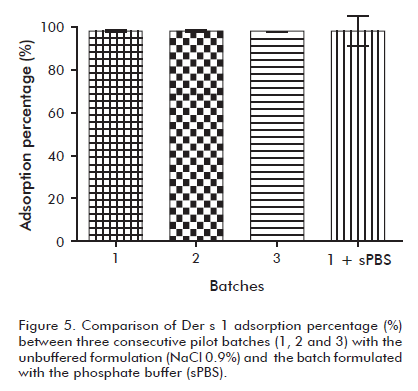
The consistency of quality parameters is a requisite of Good Manufacturing Practices (GMP) for pharmaceutical products. After confirming the negative effect of the phosphate containing buffer in the adsorption process, a fourth experiment was carried out in order to test the between-batch consistency of the adsorption process following optimal conditions, in absence of buffer.
The Der s 1 adsorption values of three consecutive pilot scale batches prepared in aseptic conditions are shown in figure 5. The obtained adsorption values were 98.7% as an average, and significantly higher than the poor adsorption reported by a batch prepared with sPBS, in aseptic conditions as well. The consistency between batches can also be appreciated in figure 5, where error bars overlap each other, indicating that there were no significant differences between the means (p < 0.05).
For testing the immunogenicity of the GMP pilot batches, as the ultimate quality criterion, an immunization schedule was performed in mice. As can be noted in figure 6 and by using the statistical multiple range test, IgG antibody titters were defined to be significantly higher at day 17 (one week after the last injection) as compared to day 0 for all groups that received the vaccine, indicating that the vaccine evidently induced the expected allergen-specific antibody response. Furthermore, the multiple sample ANOVA comparisons demonstrated that the differences between batches at day 17 were statistically not significant, thus confirming the consistency of quality parameters.
DISCUSSION
According to the first adsorption experiment, confirmed later by the third experiment, phosphate containing solution affected the adsorption process, or even desorbed already adsorbed allergen proteins. Such effect has been reported previously for other vaccine formulations containing certain proteins (8, 9) or oligonucleotides (10). In agreement with this result AP showed decreased adsorption capacity as compared to AH. Taken together, these results suggest that electrostatic attraction could be the main mechanism of adsorption of Der s 1 to alum adjuvants, since AP and Der s 1 would have the same charge at neutral pH. One possible explanation of a decreased adsorption in presence of phosphate ions, would be the displacement of hydroxyl groups by phosphate ions, leading to the partial transformation of AH into aluminum hydroxyphosphate (8, 9), leading to a decrease of the PZC, and therefore, decreasing the ability to adsorb Der s 1.
According to the last adsorption experiment and the immunization schedule, the results obtained using non-buffered saline solutions showed high and consistent adsorption levels and the vaccine showed to be immunogenic in mice. Buffers are commonly used in vaccine formulations in order to maintain the pH unchanged, assuring thus, the chemical stability of the formulation. Particularly, for injectable drug products buffers should be compatible with physiological medium. In contrast, our results showed no advantage in using phosphate buffered saline, a very common buffer used for human vaccines. Nevertheless, real time stability studies should be performed later in order to look for possible changes in pH values. Another possibility would be to investigate other buffers compatible for the injection route.
Adsorption values found in our work (over 98%) are regarded as very suitable as compared to quality specifications of marketed allergen vaccines, which usually establish a limit of at least 90% adsorption (11). The efficacy of this formulation could be judged by the immunogenicity in terms of allergen-specific IgG production, using only two injections.
CONCLUSIONS
It is concluded that the main mechanism of the adsorption process of Der s 1 into aluminum adjuvants is the electrostatic attraction. Therefore, AH showed advantage over AP regarding the adsorption level. NaCl solution was suitable for this adsorption process, while PBS buffer showed a negative effect to the adsorption of Der s 1 allergen. The consistency of the adsorption process and vaccine immunogenicity in mice, in absence of phosphate buffer, was clearly demonstrated.
ACKNOWLEDGEMENT
We are grateful to the colleagues at the animal facility of BIOCEN that help us in the immunization study.
REFERENCES
1. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol 1998;102(4 Pt 1):558-62.
2. Morefield GL, Sokolovska A, Jiang D, HogenEsch H, Robinson JP, Hem SL. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 2005;23:1588-95.
3. Gupta RK. Aluminum compounds as vaccines adjuvants. Adv Drug Deliv Rev 1998;32:155-72.
4. Ferrándiz R, Casas R, Dreborg S, Einarsson R, Bonachea I, Chapman M. Characterization of allergenic components from house dust mite Dermatophagoides siboney. Purification of Der s 1 and Der s 2 allergens. Clin Exp Allergy 1995; 25:922-8.
5. Hem SL, White JL. Structure and properties of aluminum-containing adjuvants. In: Vaccine Design: the Subunit and Adjuvant Approach. Powell MF and Newman MJ (editors), New York: Plenum Press, 1995, p. 249-76.
6. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.
7. Sewer M, Uyema K, Labrada M, Coca MA, González M. Monoclonal Antibodies against Der s 1, a Major Allergen of Dermatophagoides siboney. Int Arch Allergy Immunol 2000;123:242-8.
8. Vessely C, Estey T, Randolph TW, Henderson I, Nayar R, Carpenter JF. Effects of solution conditions and surface chemistry on the adsorption of three recombinant botulinum neurotoxin antigens to aluminum salt adjuvants. J Pharm Sci 2006; 96:2375-89.
9. Rinella JV Jr, White JL, Hem SL. Treatment of aluminum hydroxide adjuvant to optimize the adsorption of basic protein. Vaccine 1996; 14(4):298-300.
10. Aebig JA, Mullen GED, Dobrescu G, Rausch K, Lambert L, Ajose-Popoola O, et al. Formulation of vaccines containing CpG oligonucleotides and alum. J Immunol Methods 2007;323:139-46.
11. European Pharmacopoeia, monograph 1063, Allergen Products. 3rd Edition, 1997.
Received in April, 2010.
Accepted for publication in December, 2010.
Cam Anh Nguyen, Division of Medical Biotechnology, Biotechnology Centre of Ho Chi Minh City, Vietnam. E-mail: camanh_nguyenphuoc@hcmbiotech.com.vn