Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.27 n.4 La Habana oct.-dic. 2010
RESEARCH
Screening for celiac disease in a healthy Cuban children cohort from Pinar del Río province
Pesquisaje de la enfermedad celiaca en un grupo de niños cubanos sanos de la provincia de Pinar del Río
José A Galván1, Carlos Castañeda2, Emilio A Rodríguez3, Roberto Alvarez4, Norma Turcaz2, Lidia I Novoa1, Daniel O Palenzuela1
1 Immunodiagnostics and Genomics Division, Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 / 158 and 190, PO Box 6162, Havana, Cuba
2 Section of Pediatric Gastroenterology, National Institute of Gastroenterology Street 25 / H and I, Vedado, Havana, Cuba
3 Section of Pediatric Gastroenterology, Provincial Pediatric Hospital Fernando Portilla No. 71, CP 20100, Pinar del Río, Cuba
4 Mother-Child Department, Ministry of Public Health, Cuba
ABSTRACT
Recent studies suggest that celiac disease is common in many developing countries. Taking into account the disease may be underdiagnosed in Cuba, the main objectives of this study were to assess the presence of celiac disease related to antibodies in a cohort of apparently healthy children from Pinar del Río province and to evaluate a new rapid test for detecting celiac disease antibody in blood, serum and plasma samples. A total of 595 apparently healthy children with no record of first degree relatives suffering from celiac disease, were screened for Tissue transglutaminase antibodies by one-step immunochromatographic. The results were compared with commercials ELISA kits. In the study seven subjects (1.18%) were identified as positive by immunochomatographic assay and by Celikey IgG Antibody Assay with a 100% of concordance and only five subjects (0.84%) by Celikey IgA Antibody Assay. The achievement of the intestinal biopsy was offered to all positive individuals. This study demonstrates that one-step immunochromatographic assay is an appropriate tool to detect celiac disease-associated to antibodies and provides further evidence of the prevalence of possible undiagnosed celiac disease among healthy children in Cuba.
Keywords: Celiac disease, anti-tissue transglutaminase antibodies, immunochromatographic, prevalence, biopsy, Cuba.
RESUMEN
Estudios recientes sugieren que la enfermedad celíaca es común en muchos países en desarrollo. Debido a que en Cuba existe un subregistro en el diagnóstico de esta enfermedad, el principal objetivo de este estudio fue determinar la presencia de anticuerpos asociados a la enfermedad celiaca en un grupo de niños aparentemente sanos de la provincia de Pinar del Río y además evaluar una nueva prueba de diagnóstico rápido para la detección de anticuerpos anti-transglutaminasa en sangre suero y plasma. Se estudiaron un total de 595 niños aparentemente sanos que no tenían antecedentes de familiares de primer gado con enfermedad celiaca, a todos se les realizó determinación de anticuerpos anti-transglutaminasa con un ensayo inmunocromatográfico de un solo paso. Los resultados se compararon con sistemas comerciales tipo ELISA. En el estudio 7 individuos (1.18%) fueron identificados como positivos tanto por el ensayo inmunocromatográfico como por el ensayo ELISA Celikey de detección de anticuerpos IgG para un 100% de concordancia y solo 5 sujetos (0.84%) resultaron positivos para el ELISA Celikey de detección de anticuerpos IgA. A todos los individuos que resultaron positivos por cualquiera de los tres ensayos, se les ofreció la realización de la biopsia intestinal. Este estudio demuestra el valor del ensayo inmunocromatográfico de un solo paso como una herramienta útil para la detección de anticuerpos asociados a la enfermedad celiaca y nos proporciona una prueba más de la posible prevalencia de enfermedad celiaca no diagnosticada en niños sanos en Cuba.
Palabras clave: enfermedad celiaca, anticuerpos anti-transglutaminasa, inmunocromatográfico, prevalencia, biopsia, Cuba.
INTRODUCTION
Celiac disease (CD) is the most common severe food intolerance in the Western world and is due to gluten ingestion in genetically susceptible children and adults. The conclusive diagnosis of CD is carried out by intestinal biopsy, which evidences the histological changes, a characteristic of this disease. However, serological screening methods, such as those detecting anti-tissular transglutaminase antibodies (tTGA), have gained attention being cheaper and less invasive (1, 2).
CD is now considered a public health problem worldwide. CD affects as much as 0.5% to 1.0% of European or European ancestry populations, but most cases remain undiagnosed (2). Other studies carried out in Brazil (1:681) and Argentina (1:167) brought about that CD is also frequent in Latin American countries (3, 4). New epidemiological studies have demonstrated that this disorder is also common in many developing countries , the highest CD prevalence in the world (5.6%) occurs in African population originally from western Sahara (2, 5).
An early and precise diagnosis by intestinal biopsy is required (6). In order to reduce the number of biopsies needed for a precise CD diagnosis, the Federation of International Societies of Pediatric Gastroenterology (FISPGHAN) recommends the use of serological tests to evaluate the levels of antigliadin antibodies (AGA), antiendomysium antibodies (EMA) or antitransglutaminase antibodies (tTGA) in the sera of patients (2).
In Cuba, CD has been investigated since the early 80´s by clinical inspection and intestinal biopsy (7). The diagnosis is currently carried out by clinical inspection and testing AGA, results that are confirmed by biopsy if positive. However, due to the low specificity of the AGA test, biopsies were unnecessarily carried out in a high number of patients (1, 8). Recently, a visual detection immunoassay was developed to evaluate tTGA, with current capacities for a massive screening of CD among risk groups and in the general population (1, 9-15). The aims of the study were to demonstrate the presence of possible CD in apparently healthy individuals and explore the feasibility of screening for celiac disease by means of a rapid test specially designed for the detection of antibodies in blood, serum and plasma.
MATERIALS AND METHODS
Subjects
We determine the presence of tTGA in 595 children who were 3 years old (born in the 12-month period from April 2003 to March 2004), and who lived in three municipalities of Pinar del Río province, from January 2007 to March 2007. The study group was comprised of 280 males and 315 females in an age range from 3 years to 3 years, 11 months and 29 days. Blood and serum samples were obtained from all children. The blood samples were tested immediately and sera were stored at -20ºC until testing. All the children included in the study were asymptomatic with no history of first degree relatives suffering from CD and their parents agreed previously with the terms of the informed consent.
Anti-tissue transglutaminase antibodies
The anti-tissue transglutaminase whole antibody response was tested in the blood for all subjects included in the study using a fast one-step immunochromatographic assay following the manufacturer instructions (HeberFast Line® anti-transglutaminase, Heber Biotec S.A., Havana, Cuba) (9, 10). Briefly, HeberFast Line® anti-transglutaminase assay nitrocellulose strips placed into a plastic cassette were filled with 100 mL either direct blood obtained by finger puncture or serum or plasma. After 20 min, positive samples were detected as two colored lines on the strips, one in the reactive zone and the other in the control zone. A negative assay should show only a single line in the control zone of the strip. Additionally, IgA and IgG antitransglutaminase antibodies were determined in serum by using the Celikey IgA and Celikey IgG Antibody Assay (Celikey Pharmacia & Upjohn, Freiburg, Germany). IgA and IgG antitransglutaminase antibodies were also determined in parallel as a whole antibody response with the one-step immunochromatographic assay.
RESULTS
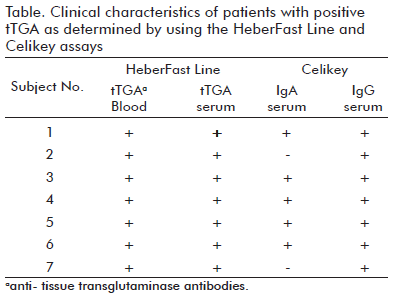
In this study, for the first time in Cuba and to our knowledge in the Caribbean region, we attempted to evaluate the prevalence of CD related antibodies among 595 (280 male/315 female) apparently healthy children, who ranged 3 years old. Only seven children (3 male and 4 female) were positive for tTGA as determined in blood by using the immunochromatographic assay. This result was further confirmed by both serum immunochromatography and Celikey Tissue Transglutaminase IgG Antibody Assay, accounting for a 1.18% seroprevalence. Among them, only five children were positive by Celikey Tissue Transglutaminase IgA Antibody Assay (see table). All the rest were consistently negative in all assays. For all tTGApositive children, a biopsy was indicated but their parents refused its achievement.
DISCUSSION
Several studies have demonstrated that CD is common in many developing countries (2-5). The presence of CD is largely established in many South American countries (3, 4) and some African countries have reported a high CD prevalence (2, 5). In the Caribbean, CD is underestimated for several reasons, for instance, the belief that this disease does not exist and the scarce diagnostic facilities. In Cuba, CD has been traditionally investigated by clinical and histological studies (7), and more recently by serological methods (1, 8-15). In previous studies, we reported the prevalence of CD in Cuba among risk groups, such as: diabetes mellitus type 1 (2.8%) (1, 14), Down´s syndrome (2.0%) (1, 13) patient with clinical symptoms (8.95%) (1) also in the "not at risk" healthy adult´s population (0.5%) (15).
In this work, we suspected the diagnosis of possible CD by sequentially determining tTGA using the immunochromatographic HeberFast Line® anti-transglutaminase assay (Heber Biotec SA, Havana, Cuba), and anti-IgA and anti-IgG antibodies with Celikey assays, respectively. Among the 595 subjects studied, seven (1.18%) were suspected of asymptomatic CD. Biopsy histological examination of distal duodenum or small intestine specimens remained the gold standard for a definitive diagnosis (2). Hence, CD diagnosis in tTGA- positive children should be confirmed by biopsy to establish the real prevalence of this disease.
It has been hypothesized that most children with CD are symptomatic early in life because of the high gluten content of infant diets (16). However, our fin ding of 1.18% seroprevalence of possible asymptomatic CD confirms reports from other authors who suggest that CD usually occurs without symptoms and in most cases remains undiagnosed (2, 17).
Additionally, we found a complete agreement between tTGA results obtained either by the immunochromatographic assay or Celikey Transglutaminase IgG, however, we did not fi nd a full consistency between tTGA results obtained with Celikey Transglutaminase IgA Antibody Assays. In fact, two patients who were negative by the Celikey Transglutaminase IgA antibody assay but positive for the HeberFast Line® and Celikey IgG antibody assay (see table). Perhaps, they could have a selective IgA deficiency because false negative results can arise due to IgA deficiency, a concomitant condition among CD patients (2, 18). On the other hand, the HeberFast Line® assay can detect both IgA and IgG antibodies in up to 20 minutes by a very simple procedure and starting from the direct samples (blood, serum or plasma) (9, 10). It has been proven as highly sensitive for CD positive samples as non-treated patients (1, 8-11). These patients must be confirmed by histological examination of biopsy specimens taken from the distal duodenum or the small intestine for definitive diagnosis (2).
In summary, this study demonstrates the presence of CD markers in a sample of apparently healthy Cuban children, from Pinar del Río Province. Our results highlight the relevance of screening for CD among the general population, also demonstrates the suitability of the HeberFast Line® anti-transglutaminase assay (Heber Biotec S.A., Havana, Cuba) as an appropriate tool for this purpose. The non invasive nature of this assay supports its application to mass screening for CD, in order to prevent medical complications in asymptomatic children.
REFERENCES
1. Sorell L, Galván JA, Acevedo B. Screening of celiac disease in Cuba. In: Catassi C, Fasano A, Corazza GR (eds.): The global village of coeliac disease. Perspectives on Coeliac Disease, vol. II. AIC Press 2005, p. 131-5.
2. Fasano A, Araya M, Bhatnagar S, Cameron D, Catassi C, Dirks M, et al. Celiac Disease Working Group. Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition Consensus report on celiac disease. J Pediatr Gastroenterol Nutr 2008;47:214-9.
3. Gandolfi L, Pratesi R, Cordoba JC, Tauil PL, Gasparin M, Catassi C. Prevalence of celiac disease among blood donors in Brazil. Am J Gastroenterol 2000;95:689-92.
4. Gomez JC, Selvaggio GS, Viola M, Pizarro B, la Motta G, de Barrio S, et al. Prevalence of celiac disease in Argentina: screening of an adult population in the La Plata area. Am J Gastroenterol 2001;96:2700-4.
5. Catassi C, Doloretta Macis M, Rätsch IM, De Virgiliis S, Cucca F. The distribution of DQ genes in the Saharawi population provides only a partial explanation for the high celiac disease prevalence. Tissue Antigens 2001;58:402-6.
6. Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) institute technical review on the diagnosis and management of celiac disease. Gastroenterology 2006;131:1981-2002.
7. Rabassa EB, Sagaró E, Fragoso T, Castañeda C, Gra B. Celiac disease in Cuban children. Arch Dis Child 1981;56:128-31.
8. Sorell L, Garrote JA, Galvan JA, Velazco C, Edrosa CR, Arranz E. Celiac Disease diagnosis in patients with giardiasis: High value of antitransglutaminase antibodies. Am J Gastroenterol 2004;99:1330-2.
9. Sorell L, Garrote JA, Acevedo B, Arranz E. One-step immunochromatographic assay for screening of coeliac disease. Lancet 2002; 359:945-6.
10. Galván JA, Acevedo B, Novoa LI, Palenzuela DO, Rubí JA, Torres E, et al. Development, validation and registration of the HeberFast Line® anti-transglutaminase system. Contribution to the diagnosis of celiac disease in Cuba. Biotecnol Apl 2008;25:66-9.
11. Cintado A, Sorell L, Galván JA, Martínez L, Castañeda C, Fragoso T, et al. HLA DQA1*0501 and DQB1*02 in Cuban celiac patients. Hum Immunol 2006;67:639-42.
12. Sánchez JC, Cabrera-Rode E, Sorell L, Galvan JA, Hernandez A, Molina G, et al. Celiac disease associated antibodies in persons with latent autoimmune diabetes of adult and type 2 diabetes. Autoimmunity 2007;40:103-7.
13. Castaneda C, Alvarez-Fumero R, Sorell L, Galvan JA, Carvajal F. Screening for celiac disease in risk groups in Cuba. J Pediatr Gastroenterol Nutr 2004;39:S211-2.
14. Galván JA, Cabrera-Rode E, Molina G, Díaz-Horta O, Palenzuela DO, Novoa LI, et al. Celiac disease-associated antibodies in type 1 diabetes patients in Cuba. Biotecnol Apl 2008;25:47-50.
15.Galván JA, Lemos G, Fernández de Cossio ME, Ruenes C, Martínez Y, Tejeda Y, et al. Silent celiac disease in a cohort of healthy adults. Autoimmunity. 2009;42:705-8.
16. Ascher H, Holm K, Kristiansson B, Mäki M. Different features of celiac disease in two neighboring countries. Arch Dis Child 1993; 69:375-80.
17. Fasano A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology 2005;128:S68-S73.
18. Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and "Club del Tenue" Working Groups on Coeliac Disease. Gut 1998;42:362-5.
Received in November, 2009.
Accepted for publication in August, 2010.