Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.28 no.4 La Habana sep.-dic. 2011
REPORT
Yeast and its derivatives as ingredients in the food industry
Las levaduras y sus derivados como ingredientes en la industria de alimentos
Miguel A Otero1, Isabel Guerrero2, Jorge R Wagner3, Agustín J Cabello1, Paula Sceni3, Roxana García1, Jorge Soriano2, Araceli Tomasini2, Gustavo Saura1, Oscar Almazán1
1Instituto Cubano de Investigaciones de los Derivados de la Caña de Azúcar, ICIDCA. Vía Blanca 804, CP 11000, La Habana, Cuba.
2Laboratorio de Investigación en Funcionalidad y Tecnología de Alimentos, LIFTA. Departamento de Ciencia y Tecnología, Universidad Nacional de Quilmes, Roque Sáenz Peña 352 Bernal - B1876BXD - Buenos Aires, Argentina.
3División de Ciencias Biológicas y de la Salud, Departamento de Biotecnología, San Rafael Atlixco 186, CP 09340, Col. Vicentina Iztapalapa, México DF.
ABSTRACT
In the last 200 years, and still today, yeast is well known for its application in brewing, alcohol fermentation and wine and bread making. They are an endless source of new food ingredients and additives with excellent functional and nutritional properties, now through the use of innovative elaboration and fractionation techniques that come mainly from biotechnology. The book reviewed here contains fourteen chapters in 246 pages that deal with yeasts employed as food ingredients and their potential as Nutraceutics. It compiles the expertise of three Latin American institutions that have given priority to the generation of basic knowledge on yeast and set the grounds for the development of new technologies based on these microorganisms. This is a sample of the alternatives offered by yeast in the fields of food science and technology.
Keywords: yeast, derivatives, food industry, nutraceutics, book.
RESUMEN
En los últimos 200 años se emplean las levaduras en la vinicultura, la fermentación alcohólica y la industria panadera. Estos microorganismos son una fuente de ingredientes nutritivos y aditivos de excelentes propiedades funcionales y nutricionales. En la actualidad ha aumentado su relevancia, con el empleo de innovadoras técnicas de elaboración y fraccionamiento, principalmente aportadas por la biotecnología. El libro referido en la presente comunicación consta de 14 capítulos que abarcan 246 páginas sobre el uso de las levaduras como ingredientes alimenticios y su potencial como nutracéuticos. En él se resume la experiencia de tres instituciones latinoamericanas que dan prioridad a la generación de conocimiento básico sobre las levaduras y que sientan cátedra para el desarrollo de nuevas tecnologías en base a estos microorganismos Este es un ejemplo de las alternativas inherentes al uso de las levaduras en los campos de las ciencias y tecnologías de la alimentación.
Palabras clave: Levadura, derivados, industria de los alimentos, nutracéuticos, libro.
INTRODUCTION
Yeast are found in a variety of habitats, from deserts to the Antarctic, sometimes commonly associated to insects, flowers, soil, plankton, etc. [1-2]. Their unlimited capacity to metabolize simple sugars as hexoses and pentoses, organic acids, phenols, hydrocarbons, etc., and their ability to produce alcohols, fats, heterologous proteins, enzymes, and a myriad of other products enable them to survive the most harsh condition in our planet [2].
The story of yeast is deeply rooted in history [3]. It is the oldest and best understood microorganism, especially the Saccharomyces genus. At about 6000 BC yeast was used in beer-like beverages, wine and bread making, by the Egyptians and centuries later by the Romans. Wine-making was confined to the Mediterranean region where a more temperate climate favored vineyard development, while the more shady forests in central Europe and its open fields offered evident advantages for cereal crops, among which barley was used for beer production [4-5]. These different forms of ethanol fermentation were spread throughout the world. In the 17th century science began to unravel yeast metabolism and the world’s first microscopes helped van Leeuwenhoek in see creatures he described as ‘animalcules’ in 1680, which were associated 150-200 years later with the biochemical observations of fermentation of sugars to alcohol.
Other scientist observed yeast under the microscope, and described the budding process of yeast cells when inoculated into a fresh medium containing sugars. Finally, Louis Pasteur in 1876 consolidated the knowledge of his time describing both fermentative – “la fermentation est la vie sans air” (i.e., fermentation is life devoid of air) – and respiratory metabolism.
Pasteur described pure culture techniques that were improved by Hansen and used in the brewing industry. The first ‘propagators’ designed by Hansen remain to this day. Somewhat later, Buchners made cell free extracts of yeast (yeast zymase) by grinding yeast with diatomaceous earth. A completely new industry soon arose, first based on amylases and proteases and their applications and later on an ample range of applications in pharmaceutical, food and environmental fields. The word enzyme was adopted to describe a protein isolated from cells of different nature that would by itself catalyze a change in a substrate to a product or products under physiological conditions. Today enzymes as well as other microbial components are isolated from plants, animals, bacteria, filamentous fungi and of course from yeasts.
The work concerning this paper received the Cuban Academy of Sciences Award in 2010.
TRADITIONAL YEAST INDUSTRIES
Saccharomyces cerevisiae yeast is used in the food and beverage industries for several applications [6]. In its active form yeast is used in bread making and bakery, in alcohol fermentation and other fermentative processes it is used, for example, in soy sauce production [5-8]. When inactivated, yeast is used in animal feeding mainly as a protein source and in human food as extracts and autolizates [9].
In modern alcohol distilleries and breweries, huge amounts of spent yeast are generated that, whether inactivated or alive, can be used directly, or processed for obtaining different derivatives [7].
Yeast cells, in addition to their high protein content, are also rich in B-complex vitamins, in minerals such as chromium and selenium, in nucleic acids and in complex carbohydrates [10].
At the end of the 20th century in Cuba the fodder yeast industry thrived, using molasses as the main carbon substrate, reaching an annual production of about 100 thousand tons through continuous culture. Although now it has significantly decreased, there is still an important production of fodder yeast using distillery slops as carbon source. Table 1 shows the average composition of different primary and spent yeasts in Cuba.
PRODUCTION OF FLAVOR ENHANCERS FROM YEASTS
Although 5’-nucleotides can be obtained from several raw materials such as hydrolizates from plant and animal proteins, they are usually produced from yeast biomass at a commercial scale. Yeasts have about 10% nucleic acids, which are an excellent source of these compounds.
The integration of these products to the resulting yeast biomass results in an enriched extract. For yeast extract production both primary and spent yeasts have been used as raw material. The cytoplasm content is released by autolysis or aided-hydrolysis if exogenous enzymes are employed (Figure 1).
Autolysis time can be significantly reduced if cell disintegration is applied together with enzyme activation [9, 10]. Usually, autolysis takes place at 50 °C during 18-24 hours. All internal or external enzymes (when they are used), are inactivated by heating the entire product at 70-85 °C once autolysis is completed. Finally the supernatant is separated by centrifugation (extract) and concentrated by evaporation. Extract commonly contains bitter peptides, thus if a milder flavor is needed, they can be eliminated with activated carbon. Obtaining 5’-mononucleotides requires the action of 5’-fosfodiestearase, since with 3’-mononucleotides the results of yeast ribonuclease action, do not have flavor-enhancing properties.
YEAST PIGMENTS
Certain yeast species such as Phaffia rhodozima, Rhodotorula gracilis, etc., are rich in pigments of carotene nature and they have therefore been used for their intensive production [11, 12]. Carotenes are important since they confer an attractive color to certain foods while also being precursors of vitamin A. On the other hand, it has been demonstrated that carotenoids have antioxidant activity that can prevent the action of free radicals on living cells. They enhance the immune system, exert protection against cancer and play an important role in the prevention of degenerative diseases.
FUNCTIONAL AND THERMAL PROPERTIES OF YEAST PROTEINS
Functional properties are those physic and chemical properties that help enhance or stabilize rheological and sensorial characteristics of a food system. These properties can be classified as: hydration properties (water retention, water absorption, solubility), structural (gelling, coagulation, film formation), surface (emulsion, foaming, lipid interaction) and organoleptic (flavor, aroma, texture).
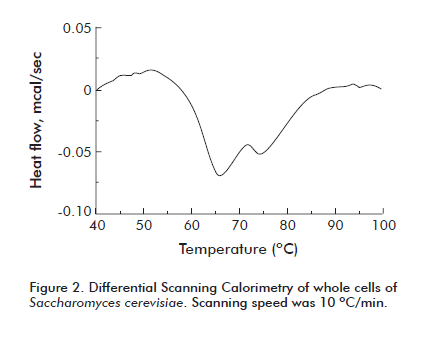
There is much technical literature on functional properties of several protein sources as milk, meat and plants. In contrast, studies on functional properties of yeast proteins are scarce. The yeast proteins more widely studied come from the genus Saccharomyces and Kluyveromyces, as well as, the specie Candida utilis, since those yeasts are generally regarded to be safe as food. Hydration and gelling properties are brought about by cytoplasm proteins mainly nuclear proteins whereas those showing the best surface properties come from membrane and cell wall lipoproteins and mannoproteins [13, 14]. Figure 2 shows the thermograms of whole S. cerevisiae yeast cells [13]. In a previous paper [14] thermograms were made with lyophilized biomass and a single protein denaturation peak appeared for both yeast species. When the analysis was carried out with active cells, an additional peak was observed in the thermogram. The main denaturation peak did not change its temperature i.e. about 65 °C. Regarding denaturation enthalpy, it can be seen that this value is higher in certain fractions formed by external proteins of cellular structure and those appearing in complexes polysaccharides or lipids. The fractions formed mainly by cytoplasm proteins and nuclear proteins, showed Tp and ΔH values that were quite similar to those of isolated yeast proteins reported in previous papers. In any case, this means that yeast proteins, even when processed as whole cells, cannot be heated beyond 60-70 °C if native proteins are needed for a certain purpose.
The thermodynamic parameters for denaturation Saccharomyces cerevisiae and Kluyveromyces fragilis isolated proteins and their peak temperatures are shown in Table 2.
When yeast proteins were isolated from their cellular environment, a higher thermal sensitivity is shown. S. cerevisiae and K. fragilis proteins have at 47 °C the same denaturation constant as K. fragilis at 57 °C and S. cerevisiae at 63 °C. It is interesting to notice that temperatures in the range of 50-65 °C are the same when applied for the activation of nucleolytic and proteolytic enzymes during autolysis processes and they can provoke high levels of denaturation depending on processing time, yeast nature and the cell disruption achieved. Nevertheless, it seems that proteins from S. cerevisiae were less affected by temperature changes that their homologues from K. fragilis. Ea and z were calculated by linear regression from the graph ln ß/TP versus 1/TP at different ß values.
YEAST SACCHARIDES
Yeasts are able to produce saccharides in a wide variety of molecular weights. Two examples are trehalose, a disaccharide, and the oligosaccharides glucans and mannans. Trehalose is a highly stable compound, formed by two glucose units. It is commonly found in insects, crustacean, honey, mushrooms, and it can be produced by yeast during fermentation. Trehalose production was highly correlated to stress, mainly osmotolerance. It is assumed that trehalose is directly related to viability of other living organisms, since this compound acts as a protective agent against damage produced by cold or dryness. Due to its multifunctional capacity, trehalose is a promising food additive. It has 45% sucrose sweetness capacity, is able to release flavors and stabilize proteins. It also improves texture and flavor in a wide variety of food products due to its humectant ability, being used for breads, confectionery, soft drinks and ice cream. However, its main characteristic is its ability to protect biological molecules against freezing; it is therefore used as an additive in foods undergoing freezing and drying processes.
Yeast cells are surrounded by special walls that are responsible for the rigidity of cell shape and the protection of the cell membrane and other inner structures. The cell wall may account for up to 20% of cell weight and is composed of glucan, mannan, and smaller amounts of other compounds. Glucan is able to bind water and has been used in the food industry as a water retention additive, thickening agent, etc. In recent years, glucans have been especially attractive because they improve the immunological system of animals and humans. Table 3 shows the results obtained with K. fragilis glucan fractions used as vaccine adjuvants.
Mouse serum typically contains 8.7-10 mg/mL of IgG. Yeast polysaccharide fractions, especially alkali-extracted fractions, induce the highest concentration of immunoglobulins in Balb/c mice. The titers obtained in our studies were significantly higher in some fractions than the levels mentioned above and those induced by a commercial adjuvant traditionally used in vaccine production.
CONCLUSIONS
The content of this paper corresponds to a book that, with 14 chapters and 246 pages, reveals the technological culture of the utilization of yeast biomass. It constitutes, in itself, a work of scientific impact, but it goes beyond the mere academic result by outlining practical applications behind the theory. This has been materialized by gathering, in a single volume, all the experience of three Latin American institutions working in this very specialized field of knowledge for more than 20 years, thereby setting forth new approaches for known problems in a different social and economic scenarios.
REFERENCES
1. Sláviková E, Vadkertiová R. The diversity of yeasts in the agricultural soil. J Basic Microbiol. 2003;43(5):430-6.
2. Spencer JFT, Spencer DM, editors. Yeasts in natural and artificial habitats. Nueva York: Springer-Verlag; 1997.
3. Barnett JA. Beginnings of microbiology and biochemistry: the contribution of yeast research. Microbiol. 2003;149(Pt 3):557-67.
4. Belcher A The world looks to higher technology to advance fuel ethanol production into the 21st Century. Int Sugar J. 2005;103(1275):196-9.
5. Corran HS, editor. A History of Brewing. Newton Abbot: Davis & Charles; 1975.
6. Halasz A, Lásztity R. Use of yeast biomass in food production. Boca Raton: CRC Press; 1991.
7. Otero MA, Cabello AJ. Procesamiento de levadura para la obtención de derivados. Diferentes alternativas. Revista ICIDCA sobre los derivados de la caña de azúcar. 2007;41(1):3-12.
8. Otero MA, Cabello AJ, Vasallo MC, Garcia L, Lopez JC. Preparation of an imitation soy sauce from hydrolyzed dried yeast Candida utilis. J Food Process Preserv. 1998;22:419-32.
9. Chae HJ, Joo H, In MJ. Utilization of brewer’s yeast cells for the production of food-grade yeast extract. Part 1: Effects of different enzymatic treatments on solid and protein recovery and flavor characteristics. Bioresour Technol. 2001;76(3):253-8.
10. Otero MA, Saura G, Martínez JA. Enriquecimiento de biomasa de levadura con nutrientes esenciales. Revista ICIDCA sobre los derivados de la caña de azúcar. 2008;42(1-3):60-8.
11. de la Fuente JL, Rodríguez-Sáiz M, Schleissner C, Díez B, Peiro E, Barredo JL. High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous. J Biotechnol. 2010;148(2-3):144-6.
12. Malisorn C, Suntornusk W. Improved B-carotene production of Rhodotorula glutinis in fermented radish brine by continuous cultivation. Biochem Eng J. 2009;43:27-32.
13. Sceni P, Palazzolo GG, Vasallo MC, Puppo MC, Otero MA, Wagner JR. Thermal and surface behavior of yeast protein fractions from Saccharomyces cerevisiae. LWT Food Sci Technol. 2009;42(6):1098-106.
14. Vasallo MC, Puppo MC, Palazolo GG, Otero MA, Beress L, Wagner JR. Cell wall proteins of Kluyveromyces fragilis. Surface and emulsifying properties. LWT Food Sci Technol. 2006;39(7):729-39.
15. Osawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2:301-24.
16. Alves-Araújo C, Almeida MJ, Sousa MJ, Leão C. Freeze tolerance of the yeast Torulaspora delbrueckii: cellular and biochemical basis. FEMS Microbiol Lett. 2004; 240(1):7-14.
Miguel A Otero. Instituto Cubano de Investigaciones de los Derivados de la Caña de Azúcar, ICIDCA. Vía Blanca 804, CP 11000, La Habana, Cuba. E-mail: maorambla@yahoo.es.