Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.29 no.4 La Habana oct.-dic. 2012
RESEARCH
Histopathological findings in egg-laying hens infected with avian infectious bronchitis virus
Hallazgos histopatológicos en gallinas ponedoras afectadas por el virus de la bronquitis infecciosa aviar
Manuel Colás1, Ana M Acevedo2, Alejandro Merino3, María del C Lamazares3, Yaima Burgher2, Julia Noda1, Dasha Fuentes2
1 Laboratorio de Investigación y Diagnóstico Aviar, Lida, Instituto de Investigaciones Avícolas, IIA. Ave. 361, No. 16632 entre 166ª y 184, Reparto Mulgoba, Boyeros, CP 19290, La Habana, Cuba.
2 Laboratorio de Virología Animal, Centro Nacional de Sanidad Agropecuaria, Censa. Apartado 10, San José de las Lajas, Mayabeque, Cuba.
3 Facultad de Medicina Veterinaria, Universidad Agraria de La Habana Fructuoso Rodríguez Pérez. Carretera de Tapaste y Autopista Nacional, San José de las Lajas, Mayabeque, Cuba.
ABSTRACT
In order to dissect the histopathological changes produced by the infection of avian infectious bronchitis virus in previously vaccinated egg-laying hens from a poultry farming unit, 35 White Leghorn egg-laying hens that had been in production for 9 to 10 months (twenty seven of which had clinical symptoms corresponding to respiratory disease and eight apparently healthy individuals) were selected for further study. After clinical examination and necropsy, they were classified into apparently healthy, mild, moderate or severe according to the severity of the clinical-pathological process. Samples were taken from paranasal sinuses, trachea and lungs for histopathological study, and trachea-lung pools were prepared from four individuals for virus isolation and molecular biology assays. The presence of mucus was evidenced with Schiff’s non-enzymatic histochemical staining, and histomorphometric analyses were used to estimate the number of glands in the tracheal mucosa. The proportions of histopathological lesions were compared, using one-way Anova to determine gland loss at the tracheae with a significance level of p < 0.05 in both cases. Histopathological analysis of the epithelia of paranasal sinuses, trachea and bronchia revealed the presence of epithelial erosion, mucous exudate and hyperplasia of mucosa-associated lymphoid tissue. Glandular cysts were observed at the paranasal sinuses, and epithelial metaplasia was detected in the trachea. It was possible to isolate and identify infectious bronchitis coronavirus from the original samples and from samples passaged in chicken embryos.
Keywords: glandular atrophy, avian infectious bronchitis, epithelial metaplasia, bronchus-associated lymphoid tissue.
RESUMEN
Con el objetivo de determinar los cambios histopatológicos causados por el virus de bronquitis infecciosa aviar en gallinas ponedoras vacunadas, afectadas con síndrome respiratorio crónico, en una unidad avícola, se seleccionaron 35 gallinas White Leghorn, entre 9 y 10 meses de postura (27 con diagnóstico clínico presuntivo de enfermedad respiratoria y ocho sin alteraciones aparentes. Se les realizó el examen clínico y la necropsia, y se clasificaron en cuatro grupos según el proceso clínico-patológico: leve, moderado, severo y control sano. Se tomaron muestras de los senos paranasales, la tráquea y los pulmones (histopatología) y mezclas de muestras de tráquea-pulmón, de tres y cuatro aves para el aislamiento del coronavirus de la bronquitis infecciosa y el análisis de biología molecular, respectivamente. Se aplicó la técnica histoquímica no enzimática del ácido periódico de Schiff, para demostrar la presencia de moco; y se cuantificaron las glándulas de la mucosa de la tráquea (histomorfometría). Se compararon las proporciones de las lesiones histopatológicas y se hizo un análisis de varianza de una vía para determinar las pérdidas de las glándulas en la tráquea, con un nivel de significación en ambos análisis de p < 0.05. Mediante técnicas histopatológicas, en el epitelio de los senos paranasales, la tráquea y los bronquios, se observó erosión del epitelio y exudado mucoso e hiperplasia del tejido linfoide asociado a mucosa. En los senos paranasales se observaron quistes glandulares y en la tráquea se observó metaplasia epitelial. A partir de muestras originales y pases en embrión de pollos se aisló e identificó el coronavirus de la bronquitis infecciosa.
Palabras clave: atrofia glandular, bronquitis infecciosa aviaria, metaplasia epitelial, tejido linfoide asociado a bronquio.
INTRODUCTION
Although egg-laying hens are a highly specialized hybrid species, realizing their full genetic and productive potential requires strict handling practices and almost extreme biosafety protocols and procedures that must be implemented along every stage of the production process. This situation is a consequence of, among other factors, the high potential for outbreaks of acute or chronic respiratory disease that characterizes intensive farming settings, with causative agents ranging from bacteria or avian mycoplasmas to pathogenic fungi or viruses [1, 2]. One prominent example of the latter case is that of avian infectious bronchitis virus (IBV), a gammacoronavirus belonging to the Coronaviridae family, in the order Nidovirales [3].
IBV is a highly infectious virus with a geographic distribution spanning the entire world. Infections with this virus exact a heavy economic toll on the poultry industry, as they produce severe weight loss in layer flocks and decrease egg production and quality, ultimately raising rejection rates at downstream processing plants [4, 5]. Morbility due to this cause is generally high (from 90 to 100%), but mortality is low (5%), although the latter increases in the case of nephropathogenic strains [6, 7].
The main clinical symptoms exhibited by laying hens affected by this virus include serous conjunctivitis, dyspnea and, ultimately, asphyxia. Its most important consequence is the reduction of egg production rates, which may reach 40% in chronic cases. Although productivity usually rebounds after 4 to 5 weeks, previous production levels are seldom recovered. The affected eggs are usually deformed, whitish, porous, exhibiting calcareous excrescences or even lacking the shell in rare cases. Their albumen is orangey amber, and there is no distinction between aqueous and dense zones. During mild respiratory infections it is common to detect renal alterations such as inflammation and discoloration of kidneys, presence of urate salts at the ureters (urolithiasis) and visceral gout. Nephropathogenic IBV pathotypes can cause this symptomatology [8].
Among the anatomopathological characteristics of mild cases of respiratory disease are excessive mucus, which can even become sebaceous –especially in broilers– and pulmonary congestion and opacity, with engrossed air sac walls. In severe cases there is also abundant mucus, producing severe inflammation with reddening of the tracheal rings in older chicken and asphyxia in younger individuals [9]. Histologically, there is epithelial hyperplasia and metaplasia, as well as loss of cilia, in both trachea and bronchi, and superficial cells are often engrossed. Subepithelial engrossment zones are characterized by edema and infiltration of the lamina, mainly by monocytes and lymphocytes [10].
The laboratory diagnosis of IBV requires isolating or directly detecting the virus, although serological techniques can be useful under some circumstances. Serotyping is done using hemagglutination inhibition assays, employing ELISA instead for serological diagnosis. Other techniques used for this purpose have included electron microscopy [9], assays based on monoclonal antibodies [11], viral neutralization assays [12] and, more recently, tests based on reverse transcriptase-polymerase chain reaction (RT-PCR) combined with restriction fragment length polymorphism to identify viral genotypes [13-15].
The continuous appearance and emergence of new serotypes has complicated viral diagnosis and the design of effective control and management programs, as the resulting antigenic variation decreases the cross-protection afforded by vaccine strains against field strains of distantly related genotypes or serotypes [16].
Control of IBV in many countries is achieved mainly through a combination of biosafety procedures and live or inactivated vaccines conferring a specific immune response [17]. In Cuba there are immunization programs against avian infectious bronchitis based on the application of live and inactivated vaccines in breeder and layer flocks, respectively [18, 19].
Despite the implementation of control procedures and biosafety practices, however, outbreaks of respiratory syndrome with high morbility and low mortality have continued to affect intensive poultry farming facilities.
For the above reasons, it was decided to examine the histopathological changes caused by infections of the avian bronchitis virus in vaccinated egg-laying hens affected by chronic respiratory syndrome.
MATERIALS AND METHODS
Bird selection
Thirty-five White Leghorn egg-laying hens approximately 38 weeks old were randomly selected from a poultry farming unit (twenty seven with a clinical diagnosis of respiratory disease, and eight apparently healthy birds that were used as a control group).
They were fed a balanced diet, and their handling complied with current technical guidelines and regulations of the country, in force since the decade of the 1980s [20].
Immunization schedule
The birds received three doses of live vaccine (strain H120, Massachusetts serotype) at 1, 35 and 85 days of life, following the immunization program currently used in the country [20].
Clinical examination and sacrifice of the birds
Both the clinical examination and sacrifice of the birds followed the methodology described by Sánchez [21]. Gross examinations were performed during necropsy, scoring the severity of clinical manifestations and recording existing anatomopathological lesions. The birds were then classified into four groups (apparently healthy, mild, moderate and severe) according to the severity of the clinical-anatomopathological alterations noticeable during gross examinations (Table).
Sampling and sample processing
Organs for the histopathological study
Sample fragments of paranasal sinuses, trachea and lung were extracted and stored in 4% formaldehyde in saline phosphate buffer. The paranasal sinuses samples were softened by placing them for 21 days in a decalcification solution.
Organs for the virological and molecular study
Trachea and lung fragments from three and four birds were taken and randomly pooled, per organ, in two groups of four and one of three (from 11 birds in total). The pools were stored at -20 °C in sterile 50 mL plastic tubes without culture medium until used.
Histopathological technique
Fragments of paranasal sinus, trachea and lungs stored in a solution of formaldehyde with saline phosphate buffer at 4% were processed by inclusion into paraffin, sectioning and staining with hematoxylin-eosin. In addition, they were also processed with Schiff’s non-enzymatic periodic acid histochemical staining (PAS), as described by Vacca [22].
Quantification of epithelial glands at the trachea of animals with respiratory processes of varying severity
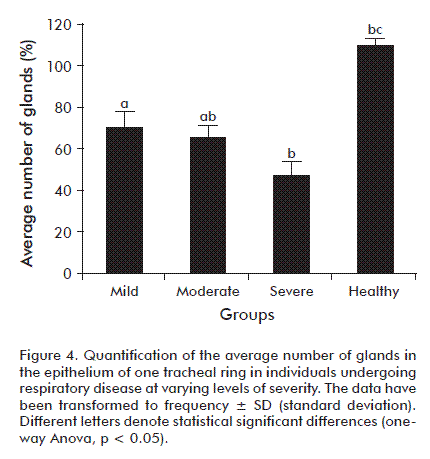
This technique employed 35 tracheal rings from all 35 birds used in the study. Gland atrophy was determined by histomorphometry of one ring from each trachea from animals falling into different levels of the chronic respiratory syndrome classification scale, based on the macroscopic clinical-anatomopathological characteristics described above.
Viral isolation and molecular identification
Samples of trachea and lungs from 11 hens in groups of three and four birds were taken after necropsy. These samples were processed and stored at -80 oC until inoculated into chicken embryos.
Organ homogenates were inoculated into 9 to 11 day-old chicken embryos, injecting 0.25 mL of the sample into the allantoic cavity and using 10 embryos for each sample. The embryos were incubated at 37 °C, and their viability was checked daily. At 72 h after inoculation, the allantoic fluid was collected, performing two to three blind passages in chicken embryos.
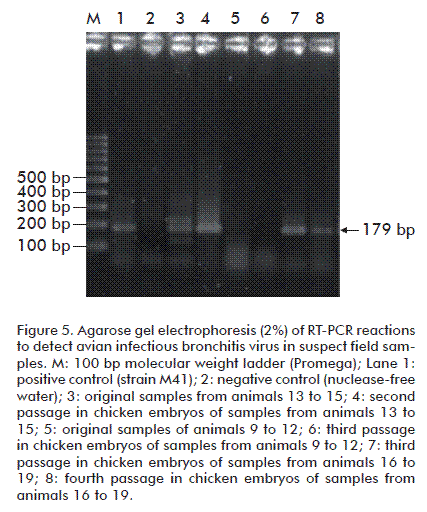
Allantoic fluid collected from each passage was evaluated using an RT-PCR assay for IBV.
Vaccine strain H120, used in the immunization program currently implemented in Cuba [20], was used as positive control.
RNA extraction
RNA was extracted using TRI Reagent® LS, following the instructions from the manufacturer (Sigma, USA). The obtained RNA was resuspended in 10 μL of nuclease-free water (Promega, USA).
RT-PCR
Viral RNA in the obtained RNA samples was detected with a semi-nested RT-PCR, using primers: sense UTR 41 - 5´ATG TCT ATC GCC AGG GAA ATG TC 3´); antisense UTR 11 - 5´ GCT CTA ACT CTA TAC TAG CCT A 3´ and UTR 31- 5´ GGG CGT CCA AGT GCT GTA CCC 3´. These primers bind to a region of the 3´ untranslated region (UTR) that is highly conserved across IBV genotypes [23].
Statistical analysis
The proportions of the principal histopathological lesions were compared, and a one-way analysis of variance (Anova) was performed to evaluate the loss of epithelial glands at the trachea, as implemented in the statistical software packages Comprop-1 and Statgraphics Plus 5.1 (Statistical Graphics Corporation, USA). A statistical significance level of p < 0.05 was chosen for both analyses.
RESULTS
The main histological changes in paranasal sinuses, trachea and lungs of laying hens, grouped according to their score in classification scheme used during gross examinations, are shown in the table.
The respiratory epithelium was markedly eroded, and there was degeneration of acinotubular glands. In individuals classified as severe there were glandular cysts with mucous exudation (Figure 1).
Another important histopathological finding is the presence of hyperplastic acinotubular glands. The latter are totally full of mucus, and secrete their contents into the lumen with some degree of distension (Figures 2A and B), as described in the table.
At the trachea there was moderate loss of cilia and hyperplasia of the bronchus-associated lymphoid tissue (BALT). In advanced stages of the respiratory infection there was also metaplasia of the cylindrical pseudostratified epithelium to flat cells, with submucosal engrossment (Figures 2C and D).
Histopathological analysis of the respiratory system also revealed changes in bronchi, such as BALT hyperplasia and a catarrhal exudative inflammatory response both in epithelial glands and the bronchial lumen (Figures 2E and F).
Upon analysis of PAS-stained sections of the respiratory epithelium of paranasal sinuses, trachea and bronchi, it was possible to confirm the presence of catarrhal exudates (mucous; figure 3).
There were statistically significant differences regarding the degree of epithelial gland loss in the tracheal rings of groups exhibiting the clinical-pathological alterations of chronic respiratory disease when compared to the control group, which had no apparent alterations. The third group (severe) exhibited the highest pathological significance (Figure 4).
The viral isolation assays employing three successive passes in chicken embryos produced symptoms not unlike those of IBV when infecting adult individuals: mortality, stunted growth and hemorrhagic embryos. Successful viral isolation was confirmed by RT-PCR analysis of clinical samples, which produced amplicons whose relative electrophoretic mobility (179 bp) matched that expected for the employed primers (Figure 5).
DISCUSSION
Avian bronchitis virus infection starts in the upper respiratory system, where it induces the secretion of mucus by goblet cells at the mucosal epithelium [24]. Most strains of this virus are able to replicate in the upper respiratory tract without producing apparent clinical signs. When clinical signs are present, the progression of lesions in this system is divided in three stages: degenerative, hyperplastic and regenerative [25]. The severity of histopathological findings paralleled the scale based on clinical signs used to classify the groups with respiratory infection (mild, moderate and severe). Some of the most conspicuous findings include the erosion of the epithelium with degenerative damage of paranasal sinus glands, BALT hyperplasia, and glandular hyperplasia with mucus hypersecretion throughout the respiratory epithelium with loss of cilia (paranasal sinuses, trachea and primary bronchi). The latter microscopic alterations, specifically those in the trachea, are defense mechanisms due to ciliary movement and the exudation of mucus by goblet cells during IBV infection [26]. These alterations, which characterize the acute stage of the disease, can be easily observed in the trachea by electron microscopy due to the anatomical simplicity of this organ [9].
Virulent strains of IBV produce epithelial damage, loss of cilia and hyperplasia. These effects predispose the individual to coinfections with opportunistic pathogens, such as Escherichia coli [27]. This enterobacterium often aggravates respiratory disease, leading in many cases to the death of infected individuals [28].
Another important histopathological finding is the presence of epithelial metaplasia, with characteristics resembling those of flat cells, and the engrossment of tracheal submucosa. These results coincide with those of an earlier study published in 2003 [29].
Controlling IBV infections through vaccination is difficult and not always successful, due to the continuous emergence of new viral serotypes and variants exhibiting very low levels of cross-protection [30, 31]. The origin of this antigenic variation is multifactorial, and includes among its causes the selective immunological pressure exerted by the widespread application of vaccines, the high frequency of coinfections –leading to recombination events as an additional source of variation– and the disappearance of once dominant serotypes due to vaccination, followed by their replacement by different field strains [32, 33].
Many different IBV vaccines -mostly against variants of the Massachusetts strain- have been developed internationally, and their efficacy in broilers and laying hens has been well studied. Most of them, however, are prone to causing the disease themselves, and the protection they provide is poor or nil [34], as reported in 1992 for the DE 072 [35, 36] and GA98 [37, 38] variants in the USA. A single group of IBV has been described in Brazil, subdivided in three subgroups together with genotype 4/91 [39-41]. Variants of the Massachusetts strains were also reported in Chile during the 1980s [42], while the Dutch serotypes (D207, D212, D3896 and D3128) have been described in Europe [43]. An IBV strain was isolated in 1980 in Africa and found to be responsible for severe respiratory problems [39, 44]. Additional Massachusetts strain variants were serotyped for Israel, during the mid 1990s [13, 45, 46] and other IBV variants were described in Korea during the mid 1980s [47]. Although a Massachusetts strain-based vaccine was used with good results in the latter case, its success was short-lived, as outbreaks of infectious bronchitis, with a high incidence of renal complications, have been taking place since 1990 in vaccinated flocks from Korea. One possible cause was uncovered by Lee et al., who found a high degree of genetic diversity among the IBV variants isolated from the diseased animals [48]. Some of these variants are indigenous, while others are genetically related to IBV variants in neighboring countries [47], suggesting that IBV strains in Korea are evolving continuously [49]. In the case of Cuba, the results suggest that other, yet to be studied variants or serotypes of the Massachusetts strain may be currently circulating.
Risk of HBV infection is also influenced by other factors, such as complete or partial vaccine coverage failures, lower vaccine efficacy against heterologous strains, presence of immunosuppressive agents, inadequate immunization schedules, improper immunization technique, variations in immunization technique (for instance, in the amount, quality and temperature of the water used to dilute the vaccine, or in the inoculated dose); and the use of vaccine combinations against different agents [50, 51].
In this work, it was possible to isolate and identify the IBV in hens, starting from the evaluation of histopathological findings in the respiratory system. Some authors state that it is not always possible to identify IBV in flocks for several reasons. One possible cause is the appearance of viral mutations, due not only to point nucleotide changes or insertions/deletions, but to recombination events between unrelated strains, whose frequency is significant due to the common occurrence of IBV coinfections in chicken [23, 52]. Although such hybrid or chimeric viruses will sometimes replicate better, the existence of differences in genetic regions is highly probable [53]. Another possible cause is the movement of flocks and the mixing of layer hen batches, which together propitiate coinfections by unrelated strains and thus, the occurrence of recombination events between separate IBV lineages.
Summarizing, the present work described the histopathological changes in the epithelium of paranasal sinuses, trachea and bronchi of egg-laying hens affected by the infectious bronchitis coronavirus, as confirmed by viral isolation in chicken embryos and identification by RT-PCR.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. C. Nelson Merino García for his support to the technical personnel of the Anatomical Pathology area during the preparation of clinical-pathological samples and for help with histopathological interpretation, as well as histologist José Suárez Alba from the Center for Genetic Engineering and Biotechnology for the H/E stains and the non-enzymatic histochemical technique used in the present study.
REFERENCES
1. Parra T. La calidad de la materia prima y el alimento terminado. Rev Acontecer Avícola. 1999;7(50):26-8.
2. Chapter 3. Vaccination. In: Alexander DJ, Bell JG, Alders RG, editors. A technology review: Newcastle disease with special emphasis on its effect on village chickens. Rome: Food and Agriculture Organization of the United Nations; 2004. p. 16-22.
3. De Groot RJ, Ziebuhr J, Poon LL, Woo PC, Talbot P, Rottier PJM, et al. Revision of the family Coronaviridae Taxonomic proposal to the Coronavirus Study Group to the ICTV Executive Committee; 2008 [cited 2011 Nov 18]. Available from http://talk.ictvonline.org/media/p/1230.aspx.
4. Wang L, Xu Y, Collisson EW. Experimental confirmation of recombination upstream of the S1 hypervariable region of infectious bronchitis virus. Virus Res. 1997;49(2):139-45.
5. Cavanagh D, Gelb J. Infectious bronchitis. In: Diseases of poultry. Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editors. Oxford: Wiley-Blackwell; 2008. p. 117-35.
6. Ziegler AF, Ladman BS, Dunn PA, Schneider A, Davison S, Miller PG, et al. Nephropathogenic infectious bronchitis in Pennsylvania chickens 1997-2000. Avian Dis. 2002;46(4):847-58.
7. Zanella A, Lavazza A, Marchi R, Moreno Martin A, Paganelli F. Avian infectious bronchitis: characterization of new isolates from Italy. Avian Dis. 2003;47(1):180-5.
8. Valencia B. Bronquitis infecciosa: soluciones prácticas [Internet]. In: Asociación de Médicos Veterinarios Especialistas en Avicultura del Ecuador, editores. Memorias Congreso Nacional de Avicultura; 2007 [cited 2010 Feb 16]. Available from: http://www.amevea-ecuador.org/memorias.htm.
9. Lamazares-Pérez MC. Algunos aspectos de la epizootiología y de diagnóstico del complejo respiratorio crónico de las aves en Cuba [dissertation]. Tesis de Doctorado. Košice: Escuela Superior de Veterinaria de Košice; 1987.
10. Jordan FTW. Bronquitis infecciosa. In: Jordan FTW, Pattison M, editors. Enfermedades de las aves. 3rd. ed. México: El Manual Moderno, S.A.; 1998. p. 171-7.
11. De Wit JJ. Detection of infectious bronchitis virus. Avian Pathol. 2000;29(2):71-93.
12. Kant A, Koch G, van Roozelaar DJ, Kusters JG, Poelwijk FA, van der Zeijst BA. Location of antigenic sites defined by neutralizing monoclonal antibodies on the S1 avian infectious bronchitis virus glycopolypeptide. J Gen Virol. 1992;73(Pt 3):591-6.
13. Meir R, Rosenblut E, Perl S, Kass N, Ayali G, Perk S, et al. Identification of a novel nephropathogenic infectious bronchitis virus in Israel. Avian Dis. 2004;48(3):635-41.
14. Stachowiak B, Key DW, Hunton P, Gillingham S, Nagy É. Infectious bronchitis virus surveillance in Ontario commercial layer flocks. J Appl Poult Res. 2005;14:141-6.
15. Grgic H, Hunter DB, Hunton P, Nagy E. Vaccine efficacy against Ontario isolates of infectious bronchitis virus. Can J Vet Res. 2009;73(3):212-6.
16. Cowen BS, Hitchner SB. Serotyping of avian infectious bronchitis viruses by the virus-neutralization test. Avian Dis. 1975;19(3):583-95.
17. Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003; 32(6):567-82.
18. Viamontes O. Impacto económico de una adecuada estrategia de inmunización contra la enfermedad de Newcastle. In: Memorias del V Congreso de Avicultura; 2006 Mar 11-13: La Habana, Cuba. La Habana: Instituto de Investigaciones Avícolas; 2006. Available from: http://www.iia.cu/congreso/seminario.htm.
19. Cuello S, Noda J, Alfonso P, Perera CL. Bronquitis Infecciosa Aviar. Cinética de anticuerpos posvacunales en reproductoras y su transferencia a la progenie. Rev Salud Anim. 2004;26(1):42-7.
20. Unión de Empresas del Combinado Avícola Nacional. Instructivo técnico para el manejo de las galinas ponedoras y sus reemplazos. La Habana: Instituto de Investigaciones Avícolas, Ministerio de Salud Pública; 2007.
21. Sánchez A. Asistencia veterinaria a unidades avícolas de producción. Sánchez A. Manual de Enfermedades de las Aves. La Habana: Editorial Félix Varela; 1990. p. 5-100.
22. Vacca LL. Laboratory manual of histochemistry. New York: Raven Press; 1985.
23. Cavanagh D, Mawditt K, Welchman Dde B, Britton P, Gough RE. Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathol. 2002;31(1):81-93.
24. Raj GD, Jones RC. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1996;26(4):677-706.
25. Cunnigham CH. Avian infectious bronchitis virus isolation and identification. International symposium on requeriments for poultry standard vaccines. Develop Boil Standard. 1974;25:211-8.
26. Rodrigo VA. Infección experimental con Escherichia coli y virus de la bronquitis infecciosa en pollos libres de patógenos específicos [dissertation]. Tesis de grado en Medicina Veterinaria; Valdivia: Instituto de Patología Animal, Universidad Austrial de Chile; 2006.
27. Peighambari SM, Julian RJ, Gyles CL. Experimental Escherichia coli respiratory infection in broilers. Avian Dis. 2000;44(4):759-69.
28. Vandekerchove D, Herdt PD, Laevens H, Butaye P, Meulemans G, Pasmans F. Significance of interactions between Escherichia coli and respiratory pathogens in layer hen flocks suffering from colibacillosis-associated mortality. Avian Pathol. 2004;33(3):298-302.
29. Brett M, De Noguera C, Quiroz C, De Rolo M, Herrera A, Martínez J. Aislamiento e identificación de tres cepas nefropatógenas del virus de bronquitis infecciosa aviar en la región central de Venezuela. Veterinaria Trop. 2003;28(1):63-77.
30. Cavanagh D, Naqi S. Infectious Bronchitis. In: Calnek BW, Barnes HJ, Beard CW. Diseases of poultry. 11th ed. Ames: Iowa State University Press; 2003. p. 101-19.
31. Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38(2):281-97.
32. Lee CW. Evolution of avian infectious bronchitis virus: genetic drift and recombination. Korean J Vet Serv. 2002;25:87-96.
33. Liu SW, Zhang QX, Chen JD, Han ZX, Liu X, Feng L, et al. Genetic diversity of avian infectious bronchitis coronavirus strains isolated in China between 1995 and 2004. Arch Virol. 2006;151(6):1133-48.
34. Wit JJ de, Cook JKA, van der Heijden HMJF. Infectious bronchitis virus in Asia, Africa, Australia and Latin America - History, current situation and control measures. Rev Bras Cienc Avic. 2010;12(2):97-106.
35. Gelb J Jr, Keeler CL Jr, Nix WA, Rosenberger JK, Cloud SS. Antigenic and S-1 genomic characterization of the Delaware variant serotype of infectious bronchitis virus. Avian Dis. 1997;41(3):661-9.
36. Lee CW, Hilt DA, Jackwood MW. Identification and analysis of the Georgia 98 serotype, a new serotype of infectious bronchitis virus. Avian Dis. 2001;45(1):164-72.
37. Jackwood MW, Hilt DA, Sellers HS, Williams SM, Lasher HN. Rapid heat-treatment attenuation of infectious bronchitis virus. Avian Pathol. 2010;39(3):227-33.
38. Hidalgo H, Gallardo R, Rosende S. Isolation of infectious bronchitis virus from broiler chickens in Chile. Avian Dis. 1976;20(3):601-3.
39. Cook JKA, Orbell SJ, Woods MA, Huggins MB. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999;28(5):477-85.
40. Di Fabio J, Rossini LI, Orbell SJ, Paul G, Huggins MB, Malo A, et al. Characterization of infectious bronchitis viruses isolated from outbreaks of disease in commercial flocks in Brazil. Avian Dis. 2000;44(3):582-9.
41. Villarreal LY, Sandri TL, Souza SP, Richtzenhain LJ, de Wit JJ, Brandao PE. Molecular epidemiology of avian infectious bronchitis in Brazil from 2007 to 2008 in breeders, broilers, and layers. Avian Dis. 2010;54(2):894-8.
42. Cubillos A, Ulloa J, Cubillos V, Cook JK. Characterisation of strains of infectious bronchitis virus isolated in Chile. Avian Pathol. 1991;20(1):85-99.
43. Davelaar FG, Kouwenhoven B, Burger AG. Occurrence and significance of infectious bronchitis virus variant strains in egg and broiler production in the Netherlands. Vet Q 1984;6(3):114-20.
44. Morley AJ, Thomson DK. Swollen-head syndrome in broiler chickens. Avian Dis. 1984;28(1):238-43.
45. Meir R, Malkinson M, Weisman Y. Characterization of IBV isolates in Israel using RT-PCR and RFLP. In: Kaleta EF, Heffels-Redmann U, editors. Proceedings of the International Symposium on Infectious Bronchitis and Pneumovirus Infections in Poultry; 1998 Jun 15-18; Rauischholzhausen, Germany. Giessen: Institut für Geflügelkrankheiten. 1998. p. 229-34.
46. Callison SA, Jackwood MW, Hilt DA. Molecular characterization of infectious bronchitis virus isolates foreign to the United States and comparison with United States isolates. Avian Dis. 2001;45(2):492-9.
47. Song CS, Lee YJ, Kim JH, Sung HW, Lee CW, Izumiya Y, et al. Epidemiological classification of infectious bronchitis virus isolated in Korea between 1986 and 1997. Avian Pathol. 1998;27(4):409-16.
48. Lee SK, Sung HW, Kwon HM. S1 glycoprotein gene analysis of infectious bronchitis viruses isolated in Korea. Arch Virol. 2004;149(3):481-94.
49. Lee EK, Jeon WJ, Lee YJ, Jeong OM, Choi JG, Kwon JH, Choi KS. Genetic diversity of avian infectious bronchitis virus isolates in Korea between 2003 and 2006. Avian Dis. 2008;52:332-7.
50. Jang JH, Sung HW, Song CS, Kwon HM. Sequence analysis of the S1 glycoprotein gene of infectious bronchitis viruses: identification of a novel phylogenetic group in Korea. J Vet Sci. 2007;8(4):401-7.
51. Jackwood MW, Hilt DA, McCall AW, Polizzi CN, McKinley ET, Williams SM. Infectious bronchitis virus field vaccination coverage and persistence of Arkansas-type viruses in commercial broilers. Avian Dis. 2009;53(2):175-83.
52. Cavanagh D, Mawditt K, Britton P, Naylor CJ. Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions. Avian Pathol. 1999;28(6):593-605.
53. Meulemans G, Boschmans M, Decaesstecker M, Berg TP, Denis P, Cavanagh D. Epidemiology of infectious bronchitis virus in Belgian broilers: a retrospective study, 1986 to 1995. Avian Pathol. 2001;30(4):411-21.
Received in November, 2011.
Accepted in June, 2012.
Manuel Colás. Laboratorio de Investigación y Diagnóstico Aviar, Lida, Instituto de Investigaciones Avícolas, IIA. Ave. 361, No. 16632 entre 166ª y 184, Reparto Mulgoba, Boyeros, CP 19290, La Habana, Cuba. E-mail: lamaz@isch.edu.cu.