Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.29 no.4 La Habana oct.-dic. 2012
RESEARCH
Genetic improvement of potato microtuber production in vitro by gamma irradiation
Mejora genética de microtubérculos de papa in vitro mediante irradiación gamma
Sherin A Mahfouze1, Amira M Esmael2, Heba Allah A Mohasseb3
1 Genetics and Cytology Department, Genetic Engineering and Biotechnology Division, National Research Center. Dokki, 12622, Egypt.
2 Tissue Culture Laboratory, Virus and Phytoplasma Research Department, Plant Pathology Research Institute, Agriculture Research Center, Giza, Egypt.
3 Plant Biotechnology Department, Genetic Engineering and Biotechnology Division, National Research Center, Dokki, 12622, Egypt.
ABSTRACT
The potato (Solanum tuberosum L.) is a major food crop in Egypt. The main problem in the program of conventional seed potato production is the low rate of multiplication in field conditions and the susceptibility of potato to diseases such as fungi, bacteria and viruses, thereby resulting in poor quality and yields. Recently, plant tissue culture technology has become very popular and has a visible impact on the production of virus free pre-basic seed potatoes. This study was aimed at producing virus free microtubers in vitro, to investigate the stimulating effects of low doses of gamma irradiation on microtuber mean number, mean fresh weight and size. Among the gamma radiation doses tested (1.5, 2, 2.5, 5 and 10 Gy), the 5 and 10 Gy doses gave the highest number of microtubers, had significant effects on microtuber weight increase and also generated the highest size microtubers (180 cm3). Additionally, nine potato unique markers were identified among the 45 polymorphic bands, as analyzed by random amplified polymorphic DNA-polymerase chain reaction profiles, with one marker detected for the 5 Gy gamma radiation dose and none for the 10 Gy. The highest number of markers (4) was obtained with the 2.5 Gy dose.
Keywords: microtuber gamma irradiation, meristem-tip, random amplified polymorphic DNA technique, Solanum tuberosum L.
RESUMEN
La papa (Solanum tuberosum L.) es uno de los cultivos más importantes de Egipto. Los principales problemas del cultivo en este país son las limitaciones en cuanto a las velocidades de multiplicación alcanzables con los programas convencionales de propagación por semillas, así como la susceptibilidad de esta planta a enfermedades fúngicas, bacterianas y virales que frecuentemente repercuten en la calidad y cantidad de las cosechas. Una alternativa, la tecnología de cultivo in vitro de tejidos vegetales, es muy popular, y ha ejercido un impacto visible en la producción de semilla prebásica libre de virus. Este estudio tuvo como objetivos la producción in vitro de microtubérculos libres de virus, así como la investigación del efecto estimulador de las dosis bajas de radiación gamma en los promedios del número de microtubérculos, peso fresco y talla. Se ensayaron varias dosis de radiación gamma (1.5, 2, 2.5, 5 y 10 Gy). Los mayores pesos, tallas (180 cm3) y cantidades se obtuvieron con dosis de 5 y 10 Gy. Adicionalmente, se identificaron nueve marcadores únicos para papa entre 45 bandas polimórficas en los patrones obtenidos por amplificación aleatoria de ADN polimórfico por PCR (RAPD), con un marcador detectado con la dosis de radiación gamma de 5 Gy y ninguno con 10 Gy. El número más alto de marcadores (4) se obtuvo con la dosis de 2.5 Gy.
Palabras clave: irradiación gamma de microtubérculos, punta del meristemo, técnica del ADN polimorfo amplificado aleatorio, Solanum tuberosum L.
INTRODUCTION
The potato (Solanum tuberosum L.) is a major food crop in Egypt after wheat, rice and maize. The main problem in the program of conventional seed potato production is the low rate of multiplication in field conditions and the susceptibility of potato to diseases, which can be transmitted through potato tubers. The risk of infection with viruses, bacteria or other pathogens increases with each multiplication of potato in the field [1, 2].
Plant tissue culture is the only technique that can eliminate viruses in tuber seed production programs and microtuber is one of the strategies in this perspective [3]. The apical meristem, together with one to three young leaf primordia, (0.1-0.5mm) used for virus elimination, has also enabled plants to be freed from other pathogens, including mycoplasmas, bacteria, and fungi [4]. Factors that affect microtuber production in vitro include growth regulators, cultivars, light quality, photoperiod and temperature. Low doses of irradiation have been reported to stimulate plant growth in vitro [5]. Radiation induced mutations have been extensively used for the improvement of crop plants. A combination of in vitro techniques and radiation induced mutagenesis has been recommended to improve cultivars of vegetatively propagated plants [6]. When the initial explant is obtained from an indexed tuber free from viruses, endogenous fungal and bacterial pathogens, the in vitro culture allows multiplication of high quality, disease-free and true-to-type tubers. The subsequent irradiation and multiplication as microtuber permits a rapid method for producing variants of standard cultivars.
The objective of the present study was to produce virus free microtubers in vitro, to investigate the stimulating effects for low doses of gamma irradiation on mean number, mean fresh weight and size of microtubers. In addition, changes in DNA caused by gamma irradiation compared with the control by random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) profiles were determined.
MATERIALS AND METHODS
Plant material
Tubers from Diamond potato cultivar grown in Egypt was obtained from Ministry of Agriculture, Dokki, Giza, Egypt.
Plant culture
Under aseptic conditions, potato tubers were surface-sterilized using 5% sodium hypochlorite solution for 10 min. The tubers were washed three times with sterile distilled water. The apical meristem together with one to three young leaf primordia (0.25 mm) was planted in glass tube with one segment per tube. Each tube contained 12 mL of MS-medium containing 0.1 mg/L 1-naphthaleneacetic acid, 0.5 mg/L kinetin and 2.25 gm/L phytagel, and they were incubated under 24 ºC and 16-h fluorescent light [4]. Develop-ing shoots from Diamond cultivar were multiplied several times to obtain enough plants to conduct the experiments.
Irradiation of cultures
In order to investigate the stimulating effects of low doses of gamma irradiation on microtuber production, growing shoots were irradiated one week after culture with five doses of gamma irradiation (1.5, 2, 2.5, 5 and 10 Gy). The source of gamma irradiation used for irradiation potato was 60Co gamma cell 3500, from the Middle Eastern Regional Radioisotope Center for the Arab countries, Giza, Egypt.
DNA extraction
Gamma irradiation-treated potato plantlets of Diamond cultivar were collected and soaked in liquid nitrogen for DNA extraction using the 2% CTAB method modified by Agrawal et al. [7].
RAPD-PCR technique
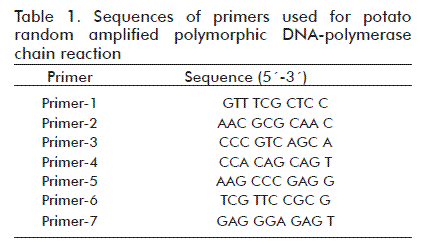
A total of seven primers (Table 1) were used to amplify DNA [8] (manufactured by Bioneer, New technology certification from ATS Korea). The total reaction mixture was 15 µL containing 10 × PCR buffer, 2 mM MgCl2, 0.2 mM dNTP mix, 10 pmol primer, 1.25 U Taq polymerase and about 150 ng genomic DNA. Amplification was obtained through 35 cycles in a DNA thermal cycler. The temperature profile was as follows: denaturing at 94 °C for 30 s; annealing at 45 °C for 1 min; and extension at 72 °C for 1 min, with a final extension at 72 ºC for 5 min. After completion of the amplification, the PCR products were separated on a 1% agarose gel containing 1 × TBE buffer (0.045 M Tris-borate, 0.001 M EDTA) and 0.5 µg/mL ethidium bromide for 45 min at 90 V. The size of each fragment was estimated with reference to a 1 kb DNA ladder marker.
Gel analysis
The gel was analyzed by using a program (UVI Geltec, version 12.4, USA).
Production of microtubers
Ten plantlets of Diamond cultivar derived from meristem-tips cultured on hormone-free MS medium with vitamins [9], and sucrose (3% w/v) as carbon source. Media were adjusted to pH 5.7. Five jar replicates were used per each treatment. The cultures were incubated at 20 ºC for 16-h daylight during 3 weeks until plantlets formation. Afterwards, the jars were taken to a laminar flow cabinet and the residual medium solution was drawn with sterilized pipette and replaced by 50 mL/jar of the tuberogenic liquid medium supplemented with 6% sucrose, 50 mg/L coumarin [10, 11]. The cultures were incubated at 20 ºC in the dark. After 10 weeks, microtubers were harvested and the number, weight and size (measured by water displacement) produced per jar were recorded.
Statistical analysis
All the experiments were arranged in a factorial completely randomized design and data were compared according to the method described by Snedecor and Cochron [12]. Analysis of variance (Anova) for all measured variables was performed using the software MSTAT-C (version 2.1). The level of significance was measured running a Duncan’s multiple range test; p ≤ 0.05 was considered as significant.
RESULTS AND DISCUSSION
Number, fresh weight and size of microtubers
Microtubers were harvested after 10 weeks from culture and the number, weight and size of microtubers produced per jar were determined (Figure 1). An Anova revealed a non-significant effect of irradiation on the number of microtubers produced in vitro. Nevertheless, the 5 and 10 Gy doses gave the highest number of microtubers. The average numbers of microtubers in jar at the 5 and 10 Gy doses measured ten weeks after culture were 17.20 and 17.00, respectively. On the other hand, the 1.5, 2 and 2.5 Gy doses had no significant effect on microtuber weight. Furthermore, the 5 and 10 Gy doses had significant effects on microtuber weight compared to the control (0 Gy), weighing 1.834 and 1.790 g, respectively. These two doses were the best in microtuber size among all irradiation treatments. The results showed that irradiation with 2, 2.5, 5 and 10 Gy doses had significant effect on microtuber size compared to the control. Once again, the 5 and 10 Gy doses gave the best results, with the highest microtuber size of 1.80 cm3 for both (Figure 1 and table 2).
Formation of potato microtubers in vitro is a complicated developmental process controlled by many factors. These factors include cultivar, growth regulators, sucrose, temperature and light. Many researchers have investigated these factors in vivo and in vitro. Pelacho and Mingo-Castel [10] found that Coumarin at 50 mg/L used to initiate tubers. The tubers obtained in this way were larger than those grown on the medium with added kinetin (2.5 mg/L). By increasing the Coumarin concentration to 100 mg/L, tuberization was delayed and the tubers were smaller. However, our study is about the effect of gamma irradiation on the induction of microtuber formation in vitro. Low doses of gamma radiation have been reported to stimulate plant growth and development, and to improve the yields and qualities of plants in vitro [13]. Irradiation of plantlets (4 Gy) led to a significant increase not only in the microtuber number (116.7 and 34.5% over the control) but also in the fresh mass (77.6 and 23.2%) in the Shepody and Atlantic potato varieties, respectively [14]. Mutation breeding is a methodology for crop improvement based on the possibility of altering genes by exposing their vegetative parts, cells, tissues, gametes or seeds to physical and chemical mutagens. Mutagenesis of in vitro cultures avoids the need for large-scale facilities and allows better control of treatment, as hyperhydric tissues may be more permeable to mutagens. The irradiation of callus cultures, which are capable of embryogenesis and organogenesis, can be used to obtain mutants quickly and in large numbers [15, 16]. Radioactive materials like 60Co emit high energy photons which are called as gamma radiation. These radiations can alter the structure of chromosomes in two ways [17]: directly by quanta of energy which hit the chromosomes like bullets hitting a target, and indirectly by ionization which produces free radicals.
RAPD-PCR analysis
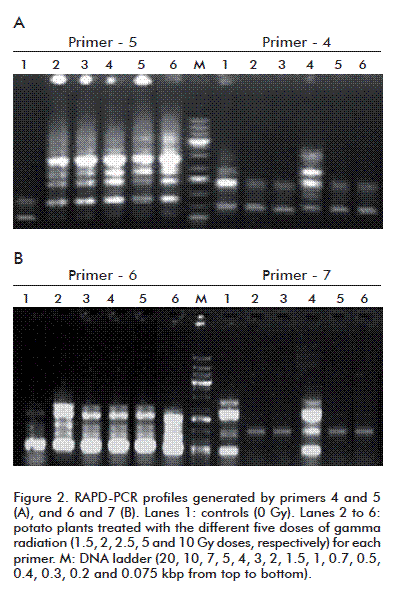
Changes in DNA caused by gamma irradiation resulting in genetic variations were detected by RAPD-PCR profiles, performed using seven arbitrary primers, which detected polymorphisms between the controls and potato plants treated with the physical mutagens (Figure 2 and table 3).
A total of 62 scorable amplified DNA fragments ranging from 128 to 21 000 bp were observed using the seven primers, whereas 45 polymorphic and 17 monomorphic bands were detected among potato plants treated with five doses of gamma radiation and the control. The seven primers showed a mean polymorphic percentage of 72.58%. The polymorphic percentage of primers 1 and 2 recorded the highest (100%), whereas primer-5 displayed the lowest percentage (30%). Among the 45 polymorphic bands, nine were unique markers with a total average of 14.52%. The potato plants markers per dose of gamma radiation varied considerably using the seven primers, with the 2.5 Gy dose showing the highest number (4), followed by 2 Gy (3) and 1.5 and 5 Gy doses (1 each), and none for the 10 Gy dose (Table 3). Ancora and Sonnino [18] used rachis, petiole and leaflet discs of potato for irradiation and observed that leaflets produced a higher number of mutated plants at lower radiation dose.
Molecular markers are important tools for precisely detecting the effects of gamma radiation since they identify genetic polymorphisms at DNA level and have been used to study genetic dissimilarity in many crop species [19-21]. For example, the inter-simple sequence repeat markers are semi-arbitrary markers, amplified by PCR using primers complementary to a target microsatellite, which have been widely used in Musa genetic diversity studies [22-24]. In those studies, the pattern quality and reproducibility of these markers have indicated that they are quick, easy to apply and highly reproducible. Rani et al. [25] found that polymorphic amplification products which represent one allele per locus can result from changes in either the sequence of the primer binding site, such as point mutations, or from changes altering the size or preventing successful amplification of a target DNA such as insertions, deletions and inversions. Traditional methods for mutant plant selection are based on morphological and biochemical markers, but these markers are less reproducible due to the influence of environmental conditions. Hence, mutation detection based on PCR and non-PCR techniques is more reliable and reproducible and have been used in various mutant crops for screening. The simplest use of PCR in mutation analysis determines the presence or absence of a particular region of DNA [26].
CONCLUSIONS
Irradiation with low doses of 5 and 10 Gy enhanced microtuber production efficiency (number, weight and size of microtubers). Gamma irradiation, as a physical mutagen, is potent, inexpensive and easy to apply on the potato plantlets in vitro to create point mutations. The mutant plant variants can be easily selected from potato plants by RAPD-PCR.
REFERENCES
1. Ranalli P, Bassi F, Ruaro G, Del Re P, Di Candilo M, Mandolino G. Microtuber and minituber production and field performance compared with normal tubers. Potato Res. 1994;37:383-91.
2. Botoman GH, Ianoşi IS. Integrated control of pests and diseases from potato crop. Bucharest: Valahia Publishing House; 2005.
3. Wang PJ, Hu CY. In vitro mass tuberization and virus-free seed-potato production in Taiwan. Am J Potato Res. 1982;59(1):33-7.
4. Edriss MH, Badawy MA, Fathi S, El-Barkouki TM. Propagation of potato using tissue culture technique. Acta Hort (ISHS). 1996;434:413-18.
5. Al-Safadi B, Simon PW. The effects of gamma irradiation on the growth and cytology of carrot (Daucus carota L.) tissue culture. Environ Exp Bot. 1990;30(3):361-71.
6. Maluszynski M, Ahloowalia BS, Sigurbjhörnsson B. Application of in vivo and in vitro mutation techniques for crop improvement. Euphytica. 1995;85(1-3):303-15.
7. Agrawal GK, Pandey RN, Agrawal VP. Isolation of DNA from Cheorospondias axillaris leaves. Biotechnol Lett. 1992;2:19-24.
8. Kang TJ, Yang MS. Rapid and reliable extraction of genomic DNA from various wild-type and transgenic plants. BMC Biotechnol. 2004;4:20.
9. Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum. 1962;15(3):473-87.
10. Pelacho AM, Mingo-Castel AM. Effect of photoperiod on kinetin-induced tuberization of isolated potato stolons cultured in vitro. Am Potato J. 1991;68:8:533-41.
11. Pruski K, Duplessis P, Lewis T, Astatkie T, Nowak J, Struik PC. Jasmonate effect on in vitro tuberization of potato (Solanum tuberosum L.) cultivars under light and dark conditions. Potato Res. 2001;44(4);315-25.
12. Snedecor GW, Cochran WG. Statistical Methods. 6th ed. Ames: The Iowa State University Press; 1967.
13. Al-Safadi B, Ayyoubi Z, Jawdat D. The effect of gamma irradiation on potato microtuber production in vitro. Plant Cell Tissue Organ Cult. 2000;61(3):183-7.
14. Li HZ, Zhou WJ, Zhang ZJ, Gu HH, Takeuchi Y, Yoneyama K. Effect of -radiation on development, yield and quality of microtubers in vitro in Solanum tuberosum L. Biol Plantarum. 2005;49(4):625-8.
15. Ziv M. Vitrification: Morphological and physiological disorders of in vitro plants. In: Debergh PC, Zimmerman RH, editors. Micropropagation: technology and application. Dordrecht: Kluwer Academic Publishers; 1991. p. 45-69.
16. Ahloowalia BS. In vitro mutagenesis for the improvement of vegetatively propagated plants. In: Induced mutations and molecular techniques for crop improvement. Proceedings, IAEA, Vienna (Austria). International Symposium on the Use of Induced Mutations and Molecular Techniques for Crop Improvement, Vienna (Austria), 19-23 Jun 1995. Vienna: IAEA; 1995. p. 531-41.
17. Ali A, Naz S, Sarwar Alam S, Iqbal J. In vitro induced mutation for screening of red rot (Colletotrichum falcatum) resistance in sugarcane (Saccharum officinarum). Pakistan J Bot. 2007;39(6):1979-94.
18. Ancora G, Sonnino A. In vitro induction of mutation in potato. In: Bajaj YPS, editor. Biotechnology in agriculture and forestry. Vol 3. Potato. Berlin: Springer; 1987. p. 408-24.
19. Souframanien J, Pawar SE, Rucha AG. Genetic variation in gamma ray induced mutants in blackgram as revealed by RAPD and ISSR markers. Indian J Genet Plant Breed. 2002;62:291-5.
20. Roy A, Bandyopadhyay A, Mahapatra AK, Ghosh SK, Singh NK, Bansal KC, et al. Evaluation of genetic diversity in jute (Corchorus species) using STMS, ISSR and RAPD markers. Plant Breed. 2006;125(3):292-7.
21. Barakat MN, Abdel Fattah RS, Badr M, El-Torky MG. In vitro mutagenesis and identification of new variants via RAPD markers for improving Chrysanthemum morifolium. Afr J Agric Res. 2010;5(8):748-57.
22. Racharak P, Eiadthong W. Genetic relationship among subspecies of Musa acuminate Colla and A-genome consisting edible cultivated bananas assayed with ISSR markers. Songklanakarin J Sci Technol. 2007;29(6):1479-89.
23. Venkatachalam L, Sreedhar RV, Bhagyalakshmi N. The use of genetic markers for detecting DNA polymorphism, genotype identification and phylogenetic relationships among banana cultivars. Mol Phylogenet Evol. 2008;47(3):974-85.
24. Pestana RKN, Amorim EP, Ferreira CF, Amorim VBO, Oliveira LS, Ledo CAS, et al. Agronomic and molecular characterization of gamma ray induced banana (Musa sp.) mutants using a multivariate statistical algorithm. Euphytica. 2011;178(2):151-8.
25. Rani V, Parida A, Raina S. Random amplified polymorphic DNA (RAPD) markers for genetic analysis in micropropagated plants of Populus deltoides Marsh. Plant Cell Rep. 1995;14(7):459-62.
26. Khan S, Al-Qurainy F, Anwar F. Sodium azide: a chemical mutagen for enhancement of agronomic traits of crop plants. Environ We Int J Sci Technol. 2009;4:1-21.
Received in February, 2012.
Accepted in July, 2012.
Sherin A Mahfouze. Genetics and Cytology Department, Genetic Engineering and Biotechnology Division, National Research Center. Dokki, 12622, Egypt. E-mail: sherinmahfouze@yahoo.com.