Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.30 no.1 La Habana ene.-mar. 2013
RESEARCH
In vitro regeneration of soybean plants of the Cuban Incasoy-36 variety
Regeneración in vitro de plantas de soya de la variedad cubana Incasoy-36
Natacha Soto, Aleines Ferreira, Celia Delgado, Gil A Enríquez
Laboratorio de Biotecnología de la Soya, División de Plantas, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11 600, La Habana, Cuba.
ABSTRACT
An efficient and reproducible plant regeneration procedure is essential for introducing genes of interest in important crops through genetic transformation. However, some crops, such as soybean [Glycine max (L.) Merrill], are difficult to manipulate in vitro, often depending on their genotype, and the reproduction of the established protocols is not always possible. The purpose of this paper is the optimization of a regeneration protocol for soybean shoots of the Cuban variety Incasoy-36 to enable its reproduction. Cotyledonary nodes of mature seeds were the explants of choice to promote regeneration under specific culture conditions. The effect of several concentrations of benzylaminopurine on shoot induction was evaluated and it was demonstrated that the age of explants is essential for regeneration. Shoot formation was increased with 1.5 mg/L of benzylaminopurine, producing a regeneration frequency of 96.8 % and 4.3 shoots in explants with a 6 day germination period. The elongation of shoots, as well as rooting occurred in an MSB5 medium without hormones. Regenerated plantlets were obtained 7-8 weeks after the start of the culture and they were morphologically similar to plants of this variety.
Keywords: Soybean, Glycine max, shoot regeneration, cotyledonary nodes.
RESUMEN
Un procedimiento eficiente y reproducible de regeneración de plantas es esencial para introducir genes de interés en cultivos importantes, mediante la transformación genética de las plantas. Sin embargo, hay cultivos como la soya [Glycine max (L.) Merrill], cuya manipulación in vitro es difícil, muchas veces depende de su genotipo, y no es posible la reproducibilidad de todos los protocolos establecidos. El objetivo de este trabajo fue la optimización de un protocolo de regeneración de brotes de soya de la variedad cubana Incasoy-36, de modo que se pueda reproducir. Para promover la inducción de los brotes en condiciones específicas de cultivo, se seleccionó el nudo cotilenodal de las semillas maduras como explant. Se evaluó el efecto de varias concentraciones of bencilaminopurina y se demostró que la edad del explant es fundamental en el proceso de regeneración. La concentración óptima para la organogénesis fue 1.5 mg/L of bencilaminopurina, que favoreció el 96.8 % de la frecuencia de formación de los brotes y una eficiencia de 4.3 brotes en explantes de 6 días de germinados. La elongación de los brotes y la inducción de las raíces ocurrieron en un medio MSB5 sin hormonas. Las plantas regeneradas se obtuvieron entre 7 y 8 semanas de iniciado el cultivo, y fueron morfológicamente similares a las de esta variedad.
Palabras clave: soya, Glycine max, regeneración de brotes, nudo cotiledonal.
INTRODUCTION
Soybean [Glycine max (L.) Merrill] is one of the most widely marketed crops and a large part of the productive land in the world is used for its cultivation. Containing high level of proteins and lipids, it is of utmost importance in human and animal feeding. Geneticists, therefore, search for methods to optimize its characteristics. Since the application of biotechnology in genetic improvement of prioritized crops, such as soybean, is based on an efficient regeneration protocol, researchers have tried to optimize the conditions to increase the regeneration of its explants. Almost all parts of the plant have been used as explants for its regeneration, either by organogenesis [1] or somatic embryogenesis [2]. These explants may be cotyledonary nodes [3, 4], stem internodes [5], epicotyl sections [6], and tissues from primary leaves [7], plumules [8], hypocotyls [9], embryogenic axes [10], immature cotyledons [11, 12], immature and mature embryos [1] and roots [13]. Through organogenesis shoots are observed after 2 to 3 months and they have the characteristics of the corresponding genotype. This contrasts with somatic embryogenesis that requires about 5 months to obtain plants, while showing a large somaclonal variation in the regenerated plants [2]. In certain organogenesis systems the explants would need two culture media, one for the introduction of the shoots and the other for their elongation. They also require more time for cultivation and more media to obtain the plants. Both the regeneration of this legume and its genetic transformation are highly dependent on the genotype of the plant. Therefore, most of the regeneration and transformation protocols established for some varieties may not be reproducible in others. The cotyledonary node is one of the most frequently used explants for genetic transformation by Agrobacterium tumefaciens [14]; and although it was used since the beginning to obtain transgenic plants [15], certain genotypes still show difficult regeneration of shoots [16-18].
Soybean production is of great importance in Cuba. Some varieties are well adapted to soil conditions and some have high yields. Here we describe an optimized protocol for organogenesis in the Cuban variety of soybean Incasoy-36 that uses the cotyledonary node of mature seeds as the explant. This procedure may be useful for in vitro multiplication, for its genetic transformation, and for the introduction of new agronomic traits.
MATERIALS AND METHODS
Plant material
Mature soybean seeds of the Cuban variety Incasoy-36, of the National institute of Agriculture Sciences (Instituto Nacional de Ciencias Agrícolas, Havana, Cuba) were used. They were disinfected with 70 % ethanol for 1 minute and then immersed in 12 % commercial sodium hypochloride for 10 min while shaking frequently. Afterwards, they were rinsed 4 times with sterile distilled water and placed to germinate in a basal medium MSB5 (MS salts [19] and vitamin B5 [20]) enriched with sucrose (30 g/L) and solidified with phytoagar (7 g/L) (Duchefa Biochemie B.V., Holland) after adjusting the pH to 5.7. The seeds were maintained 6 to 8 days at 27 ºC with a periodicity of 16 h of light and 8 h of darkness.
Preparation of the explants for the induction of shoots
The germinated seeds of 6 to 8 days were used as explants for the induction of the shoots. Each one was cut horizontally 5 to 7 mm at the hypocotyls region to eliminate the radicle. Then the cotyledons were separated through a longitudinal cut, the apical bud was eliminated and they were placed in the MSB5 medium enriched with benzylaminopurine (0.5, 1, 1.5, 2, 3 and 6 mg/L) and sucrose (30 g/L).
The pH of the medium was adjusted to 5.7 before adding the phytoagar (7 g/L). Other concentrations for the phytoagar (5, 6, 7 and 8 g/L) and phytagel (2 and 3 g/L) were tested in the medium for the induction of shoots. All explants were incubated at 27 ºC, with 16 h of light.
Twenty-one explants (7 explants/plate) were used for each treatment, and the experiments were repeated 3 times. Two parameters were evaluated, namely, the age of the explants and the concentration of the benzylaminopurine in the medium, for shoot induction. The influence of age of the explants in the regeneration of the cotyledonary node was analyzed by comparing the regeneration frequency of the explants of each age (6, 7 and 8 days) and the number of shoots per explant in the different concentrations of the benzylaminopurine.
Rooting of shoots and adaptation of plants to the soil
The regenerated shoots after 35 to 40 days (3 to 4 cm) were placed to root in the MSB5 without hormones for 7 to 15 days. The rooted plants were transferred to small plastic pots containing a mixture of organic material and zeolite (1:1 v/v), under controlled conditions of light, humidity and room temperature for 7 days. They were then transplanted to large pots and kept in a greenhouse until they flowered and produced seeds.
Statistical analysis
The cultures were periodically observed and the effect of age of the explant and concentration of benzylaminopurine in the regeneration of shoots were evaluated. The data were analyzed using a simple analysis of variance (Anova). Means were compared according to the least significant differences of Fisher (LSD) for p < 0.05. For the statistical analysis we used the Statgraphics Plus program, version 5.0.
RESULTS
Effect of age of the explant and the concentration of benzylaminopurine on the regeneration of shoots from the cotyledonary node
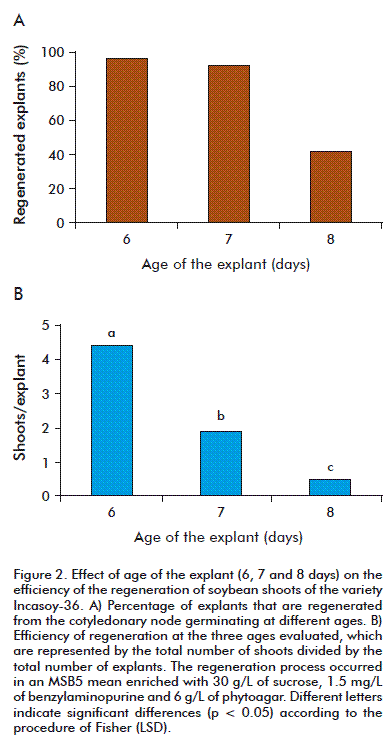
The cotyledons of mature soybean seeds germinated in vitro and selected as explants for the regeneration trials reached a green color after 6 to 8 days in the MSB5 germination medium without hormones (Figure 1). On comparing the effect of age of the explant on the regeneration of the shoots in the MSB5 medium enriched with 1.5 mg/L of benzylaminopurine, it was observed that the 6 day explants had a higher regeneration frequency of the shoots (96.8 %), although it did not differ from the frequency found in 7 day explants (92.6 %). However, the regeneration of the shoots of both ages (6 and 7 days) considerably surpassed that obtained in 8 day explants (41.2 %)(Figure 2A). On evaluating the efficiency of the regeneration, we observed similar results: the youngest explants (6 days) showed the highest number of shoots per explant and the values differed significantly from those obtained in the explants of 7 and 8 days (Figure 2B). In preliminary studies it was demonstrated that when the cotyledonary node was exposed to high concentrations of benzylaminopurine (3 to 6 mg/L), the youngest explants (6 days) showed a better regeneration response compared to explants of 7 and 8 days of germination, which developed very small shoots and much callus. The explants that did not develop shoots turned chlorotic and were dark brown at the cotyledonary node area.
The number of regenerated shoots depended on the concentration of the benzylaminopurine, although this cytokine induced organogenesis in the shoots within all tested concentrations (0.5 to 6 mg/L) (Table 1). The shoots of the explants developed at the cotyledonary node region where the cut had been made and where the axillary buds were found, after 4 weeks of culture (Figure 1B-E). The highest efficiency in the formation of shoots was obtained in the MSB5 medium with 1.5 mg/L of the compound, although the efficiency obtained did not differ from that of 2 mg/L (4.3 and 3.2 shoots/explant, respectively)(Table 1).
When the MSB5 medium was enriched with benzylaminopurine a green mass of calluses, that was compact at the cotyledonary node area, was observed in some explants where the cut was made to separate the hypocotyl (Figure 1B). This callous structure did not affect the normal growth of the shoots in concentrations lower than 2 mg/L. However, in concentrations of over 3 mg/L, the calluses were numerous in more than 30 % of the explants and the growth of shoots was then affected. Nonetheless, the shoots reached 27 % at the concentration of 6 mg/L of benzylaminopurine (Table 1). After 45 days in this medium, the explants with calluses that did not develop shoots turned dark Brown and were eliminated.
At the same time, there were multiple buds at the cotyledonary node area when the explants were in an MSB5 medium with more than 1.5 mg/L of benzylaminopurine (Figure 1F). They developed only when the cotyledonary nodes conserved the axillary buds on placing them in the induction medium with the cytokinin. When all buds were eliminated (apical and axillary buds) from the cotyledonary node, the formation of a callus mass was observed, which did not regenerate shoots. Although in the treatments with more than 3 mg/L of benzylaminopurine there was a greater presence of these multiple buds, in most of the explants they remained with an intense green color, but they did not grow (Figure 1F). Under these conditions the shoots had a well defined development (2 cm), there were very small shoots (less than 0.7 cm) and leaves. Although there were many, the very small shoots were not taken into account to determine the efficiency of regeneration. The number of shoots (of over 2 cm) was counted after 8 weeks of growth.
Effect of the solidifying agent on the morphogenesis of the cotyledonary node
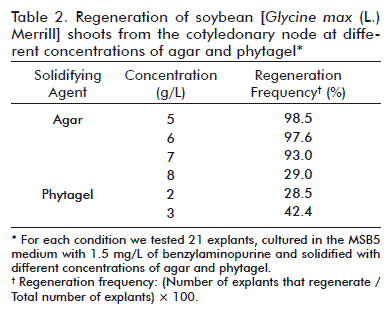
Another parameter studied was the effect of different concentrations of agar (5, 6, 7 and 8 g/L) and phytagel (2 and 3 g/L) on the formation of calluses and the regeneration of shoots from the cotyledonary node. This study was made in an MSB5 medium with 1.5 mg/L of benzylaminopurine. The best results were obtained with agar as the gelling agent (Table 2). The highest regeneration frequencies (98.5 and 97.6 %) were reached with concentrations of 5 and 6 g/L, respectively. With the four concentrations of agar well defined shoots were developed after 25 to 30 days (Figure 3A-C). However, in 8 g/L of agar, the regenerated shoots (29 %) grew slower (30 to 50 days). When using phytagel, less calluses were formed (11 and 19 %) than those of the explants cultured in agar (23 and 41 %) and the shoots had a more rapid and defined growth, which is similar to that of 5 and 7 g/L of agar (Figure 3D). In contrast, the frequency of regeneration of the shoots (28.5 and 42.4 %) did not surpass that achieved in agar.
Rooting of shoots and adaptation of plants to the soil
The regenerated shoots emerged from the cotyledonary node region, near the zone where the cut was made, without interfering in the formation of calluses (Figure 1B-E). The regenerated plants in 1.5 and 2 mg/L of benzylaminopurine presented the highest frequency of rooting (97 and 95 %, respectively; table 1). Although more than 80 % of the regenerated shoots (3 to 4 cm high) rooted after 7 to 15 days in an MSB5 medium without hormones (Figure 1 G-H), the small shoots (1 to 2 cm high) that were placed to root in this medium did not grow or develop any roots. Hence, they were transferred to an MSB5 medium enriched with 0.1 mg/L of indole acetic acid (IAA) or 0.5 mg/L of naphthalene acetic acid (NAA) to induce rooting. In these two variants we obtained a good formation of roots (Figure 1I): 96 % in the medium with IAA and 94 % in the medium with NAA. All rooted plants were transferred to the greenhouse under controlled climatic conditions and for growth. They were placed in small pots covered with a transparent plastic sheet to create a humidity chamber, and after 7 days they were transplanted to large pots for seed production (Figure 1J).
DISCUSSION
The cotyledonary node is one of the most frequently used explants for regeneration studies and the genetic transformation of soybean [15]. However, the frequency of regeneration with this type of explants is low in some varieties and the period to achieve it is long [10, 21]. The regeneration of the shoots from the cotyledonary node in this study occurred in a relatively short period (30 to 45 days) in all concentrations of benzylaminopurine tested. This confirms that it is the most effective growth regulator for the initiation of shoots [9, 21]. Several protocols use a medium to induce the formation of calluses and another one for the induction of the shoots [22, 23]. In this study we used only one medium for the induction and regeneration of the shoots and we achieved the organogenesis with well defined shoots (Figure 1 C-E). These results demonstrated that after 3 mg/L of benzylaminopurine there is a decrease in the number of shoots that are regenerated (Table 1). It has been demonstrated that high concentrations of this compound may stimulate the formation of multiple buds [24]; however, growth may be inhibited, as observed in this study. Also, high concentrations have been shown to affect the frequency of the differentiation of the shoots in Phaseolus spp. [25].
On comparing the frequency of regeneration at the three ages evaluated, we find that the regeneration response of the explants tend to decrease with age (Figure 3). The explants of 6 and 7 days reached the highest frequencies of regeneration in the MSB5 medium with 1.5 mg/L of benzylaminopurine. The 6 day old explants gave a higher regenerative response than the 7 day old explants, with all concentrations tested in this study and previous studies with the variety Incasoy-36 (Soto N; unpublished data). The juvenile tissues have a high degree of meristematic activity and tend to have more plasticity in vitro [26]. It is confirmed that the organogenic potential of an explant is inversely proportional to its physiological age [27]; therefore, the 3 day old explants showed a lower regeneration than the 6 and 7 day old explants, regardless of the concentration of the benzylaminopurine used. These results agree with those of other researchers in the in vitro regeneration of soybean varieties, in which the highest frequency of regeneration is reached in concentrations of 0.5 to 2 mg/L of benzylaminopurine [21, 24]. However, Paz et al. obtained frequencies of regeneration that were lower to those described in this study (92.6 to 96.8 %), but with a concentration of 1.12 mg/L, useing as explants cotyledons of mature seeds of the varieties: Thorne (60 %), Williams (46 %), Williams 79 (37 %) and Williams 82 (56 %) [28]. When evaluating the frequency of regeneration of the shoots from the cotyledonary node of the Cuban soybean variety, there were differences in the explants used, regardless of the hormonal concentration of the culture medium. Some explants developed many shoots, while others developed white calluses and roots; there were some that even became chlorotic and showed no morphogenesis. This demonstrates that the regenerative response in vitro tends to be variable, probably because of the endogenous levels of phytohormones during the organogenesis [26].
The formation of multiple buds in several explants was relevant. These explants coincided in that they preserved the axillary buds after cutting the cotyledonary node in half, before placing them in the regeneration medium with benzylaminopurine. Shan et al. [29] observed that the formation of multiple buds only occurred when the axillary buds are left in the cotyledonary node, while when the axillary buds are removed there is an abundant formation of calluses and no multi-buds are formed [29]. Therefore, the structural integrity of the axillary meristems contributes to the high efficiency of regeneration. Through histological studies, other researchers showed that the application of exogenous cytokinins alter the development of the axillary meristems, promote the proliferation of the meristematic cells in axillary buds and increase the number of primordial buds that are formed from the existing axillary meristems [30, 31].
Here we also obtained a low regeneration when increasing the concentration of agar in the culture medium. It is stated that high concentrations of agar create a very stressful medium for the plants, which reduces the formation of meristemoids [32]. However, some researchers have reached positive results on using 8 g/L of agar to solidify the co-culture medium [33].
We also studied the induction and development of roots in regenerated plants in vitro. In the presence of NAA, the induced roots were short (2 to 3 cm long) and thick (Figure 1I). In contrast, in the presence of IAA the roots induced were long (6 to 7 cm) and thin (Figure 1I right), and similar to those developed in the MS medium without hormones (Figure 1H). The stimulating effect of the auxins in the rooting of shoots has been described. Liu et al. described that with NAA the formation of adventitious roots were stimulated; they observed that during the induction of adventitious roots in soybean, the levels of endogenous IAA increased because of the application of exogenous NAA, which produced a greater production of adventitious roots [34]. The stimulating effect of indolebutyric acid in the increase of the number of roots induced per soybean shoot has also been reported [35].
When plants rooted in vitro are transplanted to the soil, they have a normal development and all roots are viable regardless of the medium in which they developed. This result made it possible to have other variants to achieve rooting of shoots in vitro with different states of physiological development.
Finally, a procedure was optimized to regenerate plants of the Cuban soybean variety Incasoy-36, using the cotyledonary node of mature seeds germinated in vitro, in a relatively short time and with a high frequency for shoot formation. This requires only one step to obtain the shoots and it can be used for the genetic transformation and in vitro propagation of this soybean variety.
REFERENCES
1. Barwale UB, Kerns HR, Widholm JM. Plant regeneration from callus cultures of several soybean genotypes via embryogenesis and organogenesis. Planta. 1986; 167(4):473-81.
2. Finer JJ, Nagasawa A. Development of an embryogenic suspension culture of soybean (Glycine max Merrill.). Plant Cell Tissue Organ Cult. 1988;15(2):125-36.
3. Cheng T-Y, Saka H, Voqui-Dinh TH. Plant regeneration from soybean cotyledonary node segments in culture. Plant Sci Lett. 1980;19(2):91-9.
4. Kaneda Y, Tabei Y, Nishimura S, Harada K, Akihama T, Kitamura K. Combination of thidiazuron and basal media with low salt concentrations increases the frequency of shoot organogenesis in soybeans [Glycine max (L.) Merr.]. Plant Cell Rep. 1997;17(1):8-12.
5. Kim J, LaMotte CE, Hack E. Plant Regeneration In Vitro from Primary Leaf Nodes of Soybean (Glycine max) Seedlings. J Plant Physiol. 1990;136(6):664-9.
6. Wright MS, Williams MH, Pierson PE, Carnes MG. Initiation and propagation of Glycine max L. Merr.: Plants from tissue-cultured epicotyls. Plant Cell Tissue Organ Cult. 1987;8(1):83-90.
7. Wright MS, Ward DV, Hinchee MA, Carnes MG, Kaufman RJ. Regeneration of soybean Glycine max L. Merr.) from cultured primary leaf tissue. Plant Cell Rep. 1987;6(2):83-9.
8. Yang Y-S, Wada K, Futsuhara Y. Comparative studies of organogenesis and plant regeneration in various soybean explants. Plant Sci. 1990;72(1):101-8.
9. Dan Y, Reichert N. Organogenic regeneration of soybean from hypocotyl explants. In Vitro Cell Dev-Pl. 1998;34(1):14-21.
10. Liu H-K, Yang C, Wei Z-M. Efficient Agrobacterium tumefaciens-mediated transformation of soybeans using an embryonic tip regeneration system. Planta. 2004;219(6):1042-9.
11. Samoylov VM, Tucker DM, Parrott WA. Soybean [Glycine max (L.) merrill] embryogenic cultures: The role of sucrose and total nitrogen content on proliferation. In Vitro Cell Dev-Pl. 1998;34(1):8-13.
12. Droste A, Pimentel P, Pasquali G, Mundstock E, Bodanese-Zanettini M. Regeneration of soybean via embryogenic suspension culture. Sci Agric. 2001; 58(4):753-8.
13. Bueno M, Severin C, Gattuso S, Giubileo G. Inducción de callos embriogénicos en raíces de Soja (Glycine max). Cien Inv Agr. 2004;31(1):13-9.
14. Hong H, Zhang H, Olhoft P, Hill S, Wiley H, Toren E, et al. Organogenic callus as the target for plant regeneration and transformation via Agrobacterium in soybean (Glycine max (L.) Merr.). In Vitro Cell Dev-Pl. 2007;43(6):558-68.
15. Hinchee MAW, Connor-Ward DV, Newell CA, McDonnell RE, Sato SJ, Gasser CS, et al. Production of Transgenic Soybean Plants Using Agrobacterium-Mediated DNA Transfer. Nat Biotechnol. 1988;6(8):915-22.
16. Staswick PE, Zhang Z, Clemente TE, Specht JE. Efficient down-regulation of the major vegetative storage protein genes in transgenic soybean does not compromise plant productivity. Plant Physiol. 2001;127(4):1819-26.
17. Buhr T, Sato S, Ebrahim F, Xing A, Zhou Y, Mathiesen M, et al. Ribozyme termination of RNA transcripts down-regulate seed fatty acid genes in transgenic soybean. Plant J. 2002;30(2):155-63.
18. Sato S, Xing A, Ye X, Schweiger B, Kinney A, Graef G, et al. Production of linolenic acid and stearidinic acid in seeds of marker-free transgenic soybean. Crop Sci. 2004;44(2):646-52.
19. Margulies MM. Effect of Chloramphenicol on Light Dependent Development of Seedlings of Phaseolus vulgaris var. Black Valentine, With Particular Reference to Development of Photosynthetic Activity. Plant Physiol. 1962;37(4):473-80.
20. Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50(1):151-8.
21. Ma X-H, Wu T-L. Rapid and efficient regeneration in soybean [Glycine max (L.) Merrill] from whole cotyledonary node explants. Acta Physiol Plant. 2008; 30(2):209-16.
22. Hammatt N, Davey MR, Nelson RS. Plant regeneration from seedling cotyledons, leaves and petioles of Glycine clandestina. Physiol Plant. 1986;68(1):125-8.
23. Sairam RV, Franklin G, Hassel R, Smith B, Meeker K, Kashikar N, et al. A study on the effect of genotypes, plant growth regulators and sugars in promoting plant regeneration via organogenesis from soybean cotyledonary nodal callus. Plant Cell Tissue Organ Cult. 2003;75(1):79-85.
24. Kim Y, Park T, Kim H, Park H, Chon S, Yun S. Factors affecting organogenesis from mature cotyledon explants and regeneration in Soybean. J Plant Biotechnol. 2004;6(1):39-43.
25. Malik K, Saxena P. Regeneration in Phaseolus vulgaris L.: high-frequency induction of direct formation in intact seedling by N6-benzylaminopurine and thidiazuron. Planta. 1992;186(3):384-9.
26. Litz RE, Jarret RL. Regeneración de plantas en el cultivo de tejidos: embriogénesis somática y organogénesis. In: Roca WM, Mroginski LA, editors. Cultivo de tejidos en la agricultura: Fundamentos y aplicaciones. Cali: Centro Internacional de Agricultura Tropical; 1991. p. 143-71.
27. Alleveldt G, Radler F. Interrelationship between photoperiodic behavior of grapes & growth of plant tissue cultures. Plant Physiol. 1962;37(3):376-9.
28. Paz MM, Martinez JC, Kalvig AB, Fonger TM, Wang K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006;25(3):206-13.
29. Shan Z, Raemakers K, Tzitzikas EN, Ma Z, Visser RG. Development of a highly efficient, repetitive system of organogenesis in soybean (Glycine max (L.) Merr). Plant Cell Rep. 2005;24(9):507-12.
30. Wright MS, Koehler SM, Hinchee MA, Carnes MG. Plant regeneration by organogenesis in Glycine max. Plant Cell Rep. 1986;5(2):150-54.
31. Carmen S, Ballester A, Vieitez A. Effect of thidiazuron on multiple shoot induction and plant regeneration from cotyledonary nodes of chestnut. J Hortic Sci Biotechnol. 2001;76(5):588-95.
32. Brown DCW, Leung DWM, Thorpe TA. Osmotic requirement for shoot formation in tobacco callus. Physiol Plant. 1979;46(1):36-41.
33. Paz MM, Shou H, Guo Z, Zhang Z, Banerjee AK, Wang K. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the coytyledonary node explant. Euphytica. 2004;136(2):167-9.
34. Liu ZH, Hsiao IC, Pan YW. Effect of naphthalene acetic acid on endogenous indole-3-acetic acid, peroxidase and auxin oxidase in hypocotyl cutting of soybean during root formation. Bot Bull Acad Sin. 1996;37(4):247-53.
35. Radhakrishnan R, Ranjithakumari B. Callus induction and plant regeneration of Indian soybean (Glycine max (L.) Merr. cv. CO3) via half seed explant culture. J Agric Technol. 2007;3(2):287-97.
Received in May, 2012.
Accepted in September, 2012.
Natacha Soto. Laboratorio de Biotecnología de la Soya, División de Plantas, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11 600, La Habana, Cuba. E-mail: natacha.soto@cigb.edu.cu.