Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.30 no.2 La Habana abr.-jun. 2013
RESEARCH
High-level production and aggregation of hepatitis B surface antigen in transgenic tobacco seeds
Elevada producción y agregación del antígeno de superficie de la hepatitis B en semillas de tabaco transgénico
Abel Hernández1, Alina López1, Yanaysi Ceballo1, Lissy Rosabal1, Yamilka Rosabal1, Kenia Tiel1, Marlene Pérez1, Ernesto M González2, Osmani Ramos1, Gil Enríquez1
1 Laboratorio de Biorreactores, Departamento de Plantas, Centro de Ingeniería Genética y Biotecnología, CIGB.
2 Laboratorio de purificación de proteínas, Departamento de Plantas, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
ABSTRACT
The hepatitis B surface antigen (HBsAg) has been successfully produced in tobacco leaves, potato tubers and banana fruits, but at a rather low yield. Plant seeds are well suited for the accumulation, long-time storage and preservation of recombinant proteins with pharmaceutical interest, including antigens for prophylactic or therapeutic vaccines. In this work, we assessed the efficacy of the tissue-specific phaseolin promoter in driving the expression of the HBsAg gene in tobacco seeds. Two binary constructs were designed to synthesize this antigen fused or not to the KDEL motif for protein targeting to the endoplasmic reticulum. Tobacco plants were transformed via Agrobacterium tumefaciens using each construct. The highest yield (2.2 µg of the recombinant protein per gram of seed) was achieved by a transgenic line carrying the HBsAg gene without the KDEL fusion. The heterologous antigen aggregated properly forming particles that could be determined using an immunoenzymatic assay based on monoclonal antibody which recognizes the immunodominant epitope a of HBsAg, although seed-derived antigens sedimented slightly faster than the one produced in Pichia pastoris yeast. The HBsAg-expressing tobacco seeds could constitute a novel alternative source for the cost-effective production of the antigen.
Keywords: hepatitis B surface antigen, phaseolin promoter, recombinant protein, tobacco seed.
RESUMEN
El antígeno de superficie de la hepatitis B (AgsHB) se ha producido exitosamente en hojas de tabaco, tubérculos de papa y frutas del banano, pero a muy bajos rendimientos. Se adecuan las semillas de las plantas para la acumulación, el almacenamiento y la preservación de las proteínas recombinantes con interés farmacéutico, incluyendo los antígenos para vacunas profilácticas o terapéuticas. En este trabajo se evaluó la eficacia del promotor tejido específico faseolina para dirigir la expresión del gen del AgsHB en semillas de tabaco. Se diseñaron dos construcciones binarias para sintetizarlo, fusionado o no a la señal de retención en el retículo endoplasmático KDEL. Con cada construcción se transformaron las plantas de tabaco mediante el método de Agrobacterium tumefaciens. El mayor rendimiento (2.2 µg de proteína recombinante por gramo de semilla) se alcanzó en una línea transgénica que portaba el gen del AgsHB sin la fusión de la señal KDEL. El antígeno heterólogo se agregó adecuadamente y se formaron partículas, que se detectaron mediante un ensayo inmunoenzimático basado en un anticuerpo monoclonal que reconoce el epítopo inmunodominante a del AgsHB. El antígeno producido en las semillas sedimentó ligeramente más rápido que el producido en la levadura Pichia pastoris. Las semillas de tabaco que expresan el AgsHB podrían constituir una nueva fuente alternativa para la producción económica de este antígeno.
Palabras clave: antígeno de superficie de la hepatitis B, promotor faseolina, proteína recombinante, semilla de tabaco.
INTRODUCTION
Hepatitis B virus (HBV) infection remains a major health problem, with about 350 million carriers of the virus worldwide. Several efforts are under way to control the infection, including the application of effective vaccines based on the viral surface antigen protein (HBsAg) produced in yeast. However, the costs of the vaccines available on the market are still out of reach for most of the world’s population, mainly living in poor countries.
Plant-based production of vaccines against HBV could be an attractive alternative to reduce the costs and considerably increase the affordability of these vaccines [1]. Different plant host systems have been analyzed with this purpose, including tobacco leaves [2], potato tuber [3] and banana fruit [4]. Nevertheless, plant seeds have become a focus of attention in recent years. As natural organs of high-concentration protein storage [5, 6], seeds can be envisaged as efficient minifactories of recombinant proteins. They allow for the long time storage and preservation of recombinant proteins under natural conditions [7] and have transcriptional promoters that mediate the tissue-specific expression of these proteins.
Here, the efficacy of the seed-specific phaseolin promoter in driving the expression of the HBsAg gene and the assembling of this recombinant antigen in tobacco seeds were assessed. For this purpose, two expression cassettes were designed; one of them including the sequences coding for the 2S2 signal peptide and the KDEL retrieval motif in order to relocate the antigen to the endoplasmic reticulum (ER) and the other did not include these sequences. As a result, the amount of HBsAg protein accumulated appeared to be larger than previously described in other plant systems. Furthermore, the assembly of the antigen was studied by sucrose sedimentation. To our knowledge, this is the first report on the targeted expression of HBsAg antigen in tobacco seeds.
MATERIALS AND METHODS
Plasmid constructs
Regulatory sequences of Phaseolus vulgaris storage proteins gently supplied by Proff. Ann Depicker were used to obtain the binary vectors patagHBER and patagHB (Figure 1). The pPhasG4 plasmid [5] was digested with the restriction enzymes Xho I and Nco I, to isolate the phaseolin promoter, the arcelin 5´ (arc-5I) 5´-UTR and the sequence coding for the 2S2 signal peptide. This fragment was Nco I assembled by a triple ligation together with the 654-bp Nco I-BamH I band containing the sequence of the HBsAg gene (Genbank accession number X02763) into the pSL1180 vector (Amersham Pharmacia Biotech), previously digested with the Xho I and BamH I restriction enzymes. A 300-bp fragment from pPhasG4 was amplified using sense 5´- GTGGGATCCGAACAAAAACTCATCTCAGAAGAGG-3´ and antisense 5´- TATTACGCCCGGGGGCGAAAGGGGGATG-3´ primers (the BamH I and Sma I sites are represented in bold) to add the Cmyc and KDEL coding sequences. The resulting plasmid pSLHBK, was digested with Xho I and Xba I and the 2.2-kb fragment was ligated into the Sal I and Xba I sites of patag5´-arc [5] to obtain the patagHBER plasmid.
The plasmid patagHB (Figure 1) contains the HBsAg gene under the control of the phaseolin promoter without the coding sequences for the 2S2 signal peptide and KDEL retrieval motif. To obtain it, two PCR products were amplified from pSLHBK. The first 1.4-kb fragment resulted from the amplification with sense 5´-CCCTCGAGGAATTCAATTCATT-3´ and antisense 5´- GATGTTCTCCATGGTGATCATGCAT-3´ primers (Xho I and Nco I sites in bold and the initiation codon underlined), which was subcloned at the EcoR V site of the pBluescript II SK+ vector (Stratagene, USA), resulting in the pBSphas plasmid. The second PCR product (652-bp long) was amplified with sense 5´-ACCAACGCCATGGAGAACAT-3´ and antisense 5´- TGTTCGGATCCCACTCAAATCTAT-3´ primers (Nco I and BamH I sites in bold and the stop codon underlined), and then it was ligated into pBSphas vector using the restriction sites Nco I and BamH I. Finally, the obtained plasmid was digested with Xho I and Xba I, and the 2.1-kb fragment containing the phaseolin promoter, the arc-5I UTR and the HBsAg gene was ligated to patag5´-arc, previously digested with Sal I and Xba I for the generation of the vector patagHB.
Plant transformation and propagation
The vectors patagHBER or patagHB were introduced into tobacco (Nicotiana tabacum, cv BHmN) plants using the Agrobacterium tumefaciens-mediated leaf disc method, as previously described [8]. All the kan-amycin-resistant plants were analized by PCR, and those positive for the HBsAg gene sequence were self-pollinated to obtain T2 seeds.
Protein extraction and immunoblot assay
Seed protein extracts were produced as previously described [5] with some modifications. Briefly, ground seeds were extracted three times with petroleum and ether, then lipids were removed by decantation. The residual organic solvent was evaporated using a vacuum device. Soluble proteins were extracted with freshly prepared extraction buffer (10 mM sodium phosphate, 0.15 M NaCl, 0.1 % (w/v) Tween 20, pH 7.0). The samples were incubated with gentle agitation for 1 h and then centrifuged for 10 min at 12 000 × g. The supernatant was analyzed by ELISA for the quantification of HBsAg, as previously described [9].
For the Western blot assay, 1 mL of seed extract was ultracentrifuged in a Beckman SW40Ti rotor at 30 000 × g for 30 min at 4 ºC, and the pellet fraction was resuspended in 50 µL of SDS sample buffer [10]. Proteins were separated by SDS-PAGE on a 12 % (w/v) acrylamide gel prior to transfer to a Westran polyvinylidine fluoride membrane (Whatman, USA). The membrane was blocked for 1 h with 5 % (w/v) nonfat milk (Applichem, Germany) dissolved in extraction buffer, and incubated with the anti-HBsAg monoclonal antibody CB-Hep-1 conjugated to horseradish peroxidase (Center for Genetic Engineering and Biotechnology, Cuba). Protein blots were revealed with the proper substrate. The HBsAg purified from Pichia pastoris (CIGB, Cuba) was used as standard.
Sucrose gradient analysis of recombinant HBsAg
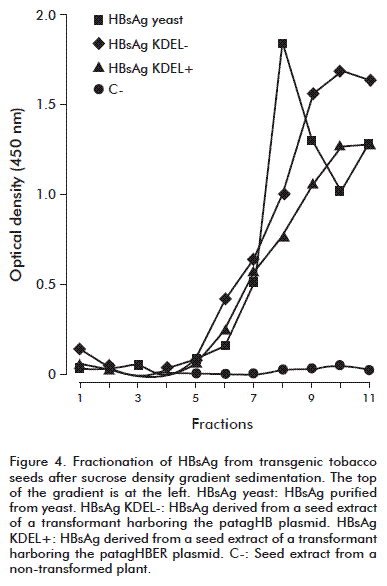
Extracts of seed tissues were obtained as described above and centrifuged at 12 000 × g. One milliliter of the supernatant was layered on discontinuous 10 mL sucrose gradients (5-50 %; w/v), made in 10 mM sodium phosphate, 0.15 M NaCl, pH 7.0. The HBsAg purified from P. pastoris was also layered on a separate gradient. The sucrose gradients were centrifuged in a Beckman SW41Ti rotor at 150 000 × g for 7 h at 4 ºC and fractionated into 1 mL aliquots. The amounts of HBsAg were determined by ELISA as described above.
RESULTS AND DISCUSSION
The HBsAg is a transmembrane protein that under goes posttranslational modifications via the secretory pathway [11]. In this study, we evaluated the effects of the fusion of a peptide containing the KDEL ER-retrieval sequence on the accumulation of HBsAg in seeds. Regulatory sequences from the storage proteins of P. vulgaris were used to direct the expression of the HBsAg gene in tobacco seeds. In contrast with other plant tissues, seeds offer a stable storage environment that could be useful also as protective delivery vehicles for orally administered vaccines or as concentrate raw material to start a protein purification procedure.
Upon genetic transformation, several tobacco plants were able to root on the selection medium. Twenty-five transgenic plants per genetic design were selected after PCR analysis, performed on genomic DNA isolated from leaves of the transformed plants (data not shown). These selected transgenic plants were allowed to produce seeds in a greenhouse.
Several studies have confirmed that transgenic plants produce HBsAg at a rather low level, as recently reviewed [12]. In this work, the HBsAg accumulated up to 2.2 µg of HBsAg per g of tobacco seeds, a level much higher than that seen in other plant systems in the literature (Figure 2). The genetic constructs patagHBER and patagHB were designed in such a way as to drive the transcription of the HBsAg gene under the control of the phaseolin promoter, one of the strongest promoters for the expression of recombinant proteins in plant seeds [13]. This amount represents a 400-fold improvement over a previous report, in which the ubiquitin 1 promoter was used to evaluate the expression of HBsAg in several tobacco organs, including seeds [14]. Nevertheless, it should be mentioned that in our case, besides the phaseolin promoter, the 5´arcelin UTR, 2S2 signal peptide and the 3´ flanking region of the arcelin 5I gene were used as part of the expression system. Using the same expression design, higher levels of accumulation of heterologous proteins have been achieved in Arabidopsis thaliana: 2-74 μg per mg of seed for different formats of antibodies [5, 6] and 4.5 μg per mg of seed for the human type I diabetes autoantigen GAD65 [15].
Noteworthy, the addition of KDEL sequence did not increase HBsAg accumulation. In fact, the best transgenic lines, which harbor the patagHB construct lacking KDEL, produced a yield about 2-fold of this antigen. Although the higher accumulation in plants of proteins containing the KDEL sequence has been extensively described [7, 16], other studies suggest that the presence of this ER-targeting signal has contrary effects on the accumulation of recombinant proteins in developing seeds [17]. Our results differ from previous works in which the incorporation of the C-terminal ER retention signal increased the accumulation of HBsAg in tobacco seeds [14]. While the size of antigen produced in seeds was similar to the 24-kDa sized produced in P. pastoris, as evidenced by Western blot of several transgenic lines harboring the patagHB construct (Figure 3), the recombinant protein in the case of the patagHBER construct was visualized with a size larger than that seen in P. pastoris cells. Additionally, of all the analyzed transgenic lines harboring the patagHBER construct, HBsAg could only be detected by Western blot analysis in the transgenic line ER19 as a band of 27 kDa.
Several degradation products were abundant in all the analyzed lines. Nevertheless, they were mainly defined bands, suggestive of sequence-specific degradation, as observed for other proteins expressed in seeds [16, 17]. This could indicate the access of the antigen to other more degradative environments beyond the ER as seed vacuoles, possibly due to saturation of KDEL receptors as previously stated [17]. Another explanation could involve the toxic behavior of the protein for the cell at high levels of accumulation in a constrained space, when the expression cassette elements are optimized for targeting seeds and particularly for secretion. These could be seen as a compensatory mechanism in plant cells for recombinant proteins (discussed in [17]). Noteworthy, the KDEL-lacking construct provided higher yields, maybe reflecting that in seeds is enough to optimize the elements of the expression cassette, unless targeting required. This can explain the higher yield obtained for other proteins when the expression is neither seed-targeted nor optimized [14], maybe by preserving the expressed protein from degradation-prone environments.
The larger size of the HBsAg derived from the transgenic plants harboring the patagHBER vector, is a result of the fusion of twenty-one aminoacids including the KDEL tetrapeptide to its C-terminus, and could have affected the accumulation profile of this antigen. Peptides and proteins did not affect the stability and assembly of HBsAg when fused to its N-terminus [18, 19]; however, this was not the case if fusion occurred at the C-terminus [19]. The HBsAg produced in seeds containing patagHBER was more susceptible to degradation, as determined by Western blot analysis. This could explain the lower accumulation of HBsAg in plants transformed with this construct as compared to plants harboring patagHB.
The capture of seed-derived HBsAg using the CB. Hep-1 monoclonal antibody and its detection by sandwich ELISA using the same antibody, suggest a multimeric structure for the HBsAg produced in seeds. This monoclonal antibody recognizes the sequence CKTC on the determinant a of the antigen [20], evidencing the correct exposure of this region. Sucrose gradient centrifugation was used to determine the putative olig-omeric assembly of the recombinant HBsAg protein (Figure 4). An analysis of the sedimentation profile of the HBsAg derived from seeds showed that it sedimented slightly faster than the produced in yeast, and thus could have a larger particle size than the yeast-derived variant. Moreover, the broader peak associated with the HBsAg obtained from the seeds suggests a wider range of particle sizes, regardless whether the recombinant protein had the peptide fused to its C-terminus or not. These data are in agreement with previous findings of HBsAg particles produced in tobacco leaves were characterized by sedimentation and electron microscopy studies [2]. Despite this difference in size, the anti-hepatitis B response to the tobacco leaves-derived HBsAg was qualitatively similar to that obtained by immunizing mice with yeast-derived HBsAg [21], suggesting that recombinant HBsAg produced in tobacco seed could be immunogenic.
Although HBsAg has been commonly expressed in plants, no study to date proposed the accumulation of this protein in the seeds of a non-edible plant. This poses ethical and regulatory advantages with respect to other seed-based expression systems. The levels of expression achieved in this work and the aggregation of HBsAg support the use of regulatory sequences derived from seed storage proteins as appropriate signals for the accumulation of proteins of pharmaceutical use in tobacco seeds, regardless of the complexity of the proteins selected. Other improvements in the tobacco seed platform to produce pharmaceutical compounds are warranted in the near future.
ACKNOWLEDGEMENTS
We thank Prof. Ann Depicker for the supply of seed expression signals and we are grateful to Dr. Alejandro Fuentes and Dr. Jorge Martin for critical reading of the manuscript.
REFERENCES
1. Kumar GB, Ganapathi TR, Bapat VA. Production of hepatitis B surface antigen in recombinant plant systems: an update. Biotechnol Prog. 2007;23(3):532-9.
2. Mason HS, Lam DM, Arntzen CJ. Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci USA. 1992;89(24):11745-9.
3. Richter LJ, Thanavala Y, Arntzen CJ, Mason HS. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol. 2000;18(11):1167-71.
4. Kumar GB, Ganapathi TR, Revathi CJ, Srinivas L, Bapat VA. Expression of hepatitis B surface antigen in transgenic banana plants. Planta. 2005;222(3):484-93.
5. De Jaeger G, Scheffer S, Jacobs A, Zambre M, Zobell O, Goossens A, et al. Boosting heterologous protein production in transgenic dicotyledonous seeds using Phaseolus vulgaris regulatory sequences. Nat Biotechnol. 2002;20(12):1265-8.
6. Van Droogenbroeck B, Cao J, Stadlmann J, Altmann F, Colanesi S, Hillmer S, et al. Aberrant localization and underglycosylation of highly accumulating single-chain Fv-Fc antibodies in transgenic Arabidopsis seeds. Proc Natl Acad Sci USA. 2007;104(4):1430-5.
7. Ramirez YJ, Tasciotti E, Gutierrez-Ortega A, Donayre Torres AJ, Olivera Flores MT, Giacca M, et al. Fruit-specific expression of the human immunodeficiency virus type 1 tat gene in tomato plants and its immunogenic potential in mice. Clin Vaccine Immunol. 2007;14(6):685-92.
8. Horsch RB, Klee HJ. Rapid assay of foreign gene expression in leaf discs transformed by Agrobacterium tumefaciens: Role of T-DNA borders in the transfer process. Proc Natl Acad Sci USA. 1986;83(12):4428-32.
9. Leyva A, Sánchez JC, López L, Font M, Gonzalez T, Pérez B, et al. Development, validation and application of a new ELISA for process control of the production of recombinant Hepatitis B surface antigen. Biotecnol Apl. 2011;28(4):228-34.
10. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680-5.
11. Eble BE, MacRae DR, Lingappa VR, Ganem D. Multiple topogenic sequences determine the transmembrane orientation of the hepatitis B surface antigen. Mol Cell Biol. 1987;7(10):3591-601.
12. Shchelkunov SN, Shchelkunova GA. Plant-based vaccines against human hepatitis B virus. Expert Rev Vaccines. 2010;9(8):947-55.
13. Boothe J, Nykiforuk C, Shen Y, Zaplachinski S, Szarka S, Kuhlman P, et al. Seed-based expression systems for plant molecular farming. Plant Biotechnol J. 2010;8(5):588-606.
14. Kumar GB, Srinivas L, Ganapathi TR, Bapat VA. Hepatitis B surface antigen expression in transgenic tobacco (Nicotiana tabacum) plants using four different expression cassettes. Plant Cell Tiss Org Cult. 2006;84(3):315-23.
15. Morandini F, Avesani L, Bortesi L, Van Droogenbroeck B, De Wilde K, Arcalis E, et al. Non-food/feed seeds as biofactories for the high-yield production of recombinant pharmaceuticals. Plant Biotechnol J. 2011;9(8):911-21.
16. Wandelt CI, Khan MR, Craig S, Schroeder HE, Spencer D, Higgins TJ. Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J. 1992;2(2):181-92.
17. Loos A, Van Droogenbroeck B, Hillmer S, Grass J, Kunert R, Cao J, et al. Production of monoclonal antibodies with a controlled N-glycosylation pattern in seeds of Arabidopsis thaliana. Plant Biotechnol J. 2011;9(2):179-92.
18. Sojikul P, Buehner N, Mason HS. A plant signal peptide-hepatitis B surface antigen fusion protein with enhanced stability and immunogenicity expressed in plant cells. Proc Natl Acad Sci USA. 2003;100(5):2209-14.
19. Huang Z, Mason HS. Conformational analysis of hepatitis B surface antigen fusions in an Agrobacterium-mediated transient expression system. Plant Biotechnol J. 2004;2(3):241-9.
20. Felici F, Puchades Y, Fernández de Cossío ME, Vispo NS. Exposición multivalente utilizando la proteína pVIII de la cápsida de los fagos filamentosos. In: Nelson Santiago Vispo, editor. Biología combinatoria. La Habana: Elfos Scientiae; 2001. p. 69-89.
21. Thanavala Y, Yang YF, Lyons P, Mason HS, Arntzen C. Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc Natl Acad Sci USA. 1995;92(8):3358-61.
Received in October, 2012.
Accepted in December, 2012.
Abel Hernández. Laboratorio de Biorreactores, Departamento de Plantas, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba. E-mail: abel.hernandez@cigb.edu.cu.