My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.30 no.2 La Habana Apr.-June 2013
RESEARCH
Preclinical safety testing of the Quimi-Hib® vaccine adjuvanted with aluminum phosphate during product development
Seguridad preclínica de la vacuna Quimi-Hib® adyuvada con fosfato de aluminio durante el desarrollo del producto
Dania Bacardí1, Karelia Cosme1, Lizet Aldana1, Nelson Merino2, José Suárez1, Omar Mosqueda1, Dioslaida Urquiza1, Juan Romero1, Roberto Madrigal1, Rubén Amaya1, Lourdes Hernández1
1 Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11 600, La Habana, Cuba.
2 Centro de Investigaciones y Desarrollo de Medicamentos, Cidem. Calle 17 e/ 62 y 64, Playa, La Habana, Cuba.
ABSTRACT
Haemophilus influenzae type b (Hib) is a Gram-negative bacterium causing diseases such as meningitis, pneumonia, epiglottis, cellulitis, septicemia and arthritis, among others. Hib-caused meningitis / Hib meningitis is a very serious disease with a death rate of more than 50 % throughout the world; therefore obtaining a vaccine against this bacterium is a goal of the highest priority. The present report presents the results of preclinical safety testing of the Quimi-Hib® vaccine (synthetic Hib antigen conjugated to tetanus toxoid) adjuvanted with aluminum phosphate. The battery of toxicological tests included acute toxicity (15 days), local tolerance (15 days) and repeated-dose toxicity (30 days), all of them evaluating the therapeutic dose of the vaccine as part of the studied experimental treatments. Three vaccine dose levels and a placebo (excipients plus adjuvant) were examined, administering the vaccine intramuscularly to Sprague Dawley rats of both sexes. After examining the data obtained from the administration of this wide range of doses to Sprague Dawley rats, it was concluded that the synthetic conjugate vaccine Quimi-Hib® adjuvanted in aluminum phosphate is not toxic and does not cause systemic adverse effects. No macroscopic alterations were observed in the studied organs, and the evaluation of the inoculation site only detected indurations caused by macrophage granulomas, resulting from the mechanism of action of the adjuvant included in the formulation.
Keywords: vaccines, synthetic vaccine, Haemophilus influenzae type b, safety, toxicity.
RESUMEN
Haemophilus influenzae tipo b (Hib) es una bacteria gramnegativa causante de enfermedades como meningitis, neumonía, epiglotitis, celulitis, septicemia, artritis, entre otras, en los seres humanos. La meningitis causada por Hib es muy grave, con una tasa de muertes de más del 50 % en varias partes del mundo. Por tales razones, la obtención de una vacuna contra esta afección es una prioridad. El objetivo de este estudio fue evaluar la seguridad preclínica de la vacuna Quimi-Hib® (antígeno sintético de Hib conjugado con toxoide tetánico), en una formulación adyuvada con fosfato de aluminio, durante la etapa de desarrollo del producto. Los ensayos toxicológicos incluyeron estudios de toxicidad aguda (15 días), de tolerancia local (30 días) y de toxicidad a dosis repetidas (15 días), que incluyeron la dosis terapéutica de la vacuna. Se exploraron tres niveles de dosis de la vacuna y un placebo (excipientes más adyuvante), que se administraron por vía intramuscular a ratas Sprague Dawley de ambos sexos. Según nuestros resultados en el espectro de dosis explorado en las ratas, se concluyó que la vacuna conjugada Quimi-Hib® adyuvada en fosfato de aluminio no es tóxica, ni provoca efectos sistémicos adversos en esa especie. No se detectaron alteraciones macroscópicas en los órganos estudiados, y al evaluar el sitio de administración, solo se observaron induraciones causadas por granulomas macrofágicos, resultantes del mecanismo de acción del adyuvante incluido en la formulación.
Palabras clave: vacunas, vacuna sintética, Haemophilus influenzae tipo b, seguridad, toxicidad.
INTRODUCTION
Haemophilus influenzae (Hib) is a pleomorphic, aerobic Gram-negative coccobacillus discovered in 1892 by Richard Pfeiffer during a flu pandemic, which colonizes the mucosae of the upper respiratory tract of three-month-old children. Most Hib isolates are non-capsulated, although some strains produce a polysaccharide capsule. This bacterium is a causative agent for meningitis, pneumonia, epiglottitis, cellulitis, septicemia, arthritis, osteomyelitis, pericarditis, cholangitis, endocarditis and sinusitis [1]. Hib meningitis is a very dangerous disease that exhibits a mortality rate of over 50 % in several parts of the world and leaves severe neurological sequelae on 50 % of the survivors [2].
Infants and toddlers are particularly vulnerable to Hib infections, as in utero-acquired antibody wanes after 3 months of age. In the absence of vaccination, natural immunity against the bacterium appears only by the fifth to sixth year of life [3, 4].
There are at least four commercially available safe and effective conjugate vaccines against H. influenzae type b approved for clinical use in several countries, prepared by chemically conjugating polyribosylribitol phosphate (PRP, corresponding to the capsular polysaccharide of H. influenzae type b) to a protein of bacterial origin. None of them, however, contains entirely synthetic antigens or are manufactured in Cuba. A collaborative effort undertaken in 1989 by the Synthetic Antigens Laboratory of the Faculty of Chemistry of the University of Havana together with the Finlay Institute and the Center for Genetic Engineering and Biotechnology (both from Havana, Cuba) resulted in the development of Quimi-Hib®, a conjugate vaccine containing 10 µg of a chemically synthesized fragment of PRP covalently linked to 20 or 30 µg of tetanus toxoid. As reference for protection in immunized children, anti-PRP titers of 1 µg/mL are used, but 0.15 µg/mL supposes an inverse correlation with disease incidence in non-vaccinated individuals [5]. An anti-Hib antibody titer of 1 µg/mL at three weeks post-immunization is predicted to provide protection for at least one year [6, 7]. The vaccine is designed to be delivered in children using doses of 10 mg injected at 2, 4 and 6 months of age.
The objective of the present investigation was to perform the preclinical safety testing of Quimi-Hib® as a vaccine candidate adjuvanted in aluminum phosphate. The assays included acute toxicity tests (15 days long), tests for repeated-dose toxicity (15 days) and local tolerance (30 days), employing three different dosages and a placebo. The therapeutic dose (TD) was examined in the local tolerance study. All tests employed Sprague-Dawley rats and used the intramuscular immunization route in order to reproduce the administration procedure planned for future clinical trials.
MATERIALS AND METHODS
Test system
Different studies predating the safety testing of this vaccine candidate determined the biological activity of PRP in different rodent and Lagomorph species. One of them used Sprague-Dawley rats, split into groups of 5 female individuals per treatment (data not shown). Each 0.5 mL dose contained 10 µg of PRP, and other immunogens under evaluation included other monovalent Hib vaccine preparations adsorbed onto aluminum phosphate, in which the amount of Hib was varied from 2 to 20 µg. This immunogenicity study validated rats once again as the premier species for toxicology testing, since the IgG antibody response obtained in these animals was higher than in other species immunized with the commercially available Hiberix® vaccine (GlaxoSmithKline).
The preclinical safety tests described in the present work for the Quimi-Hib® vaccine adjuvanted in aluminum phosphate were performed on Sprague-Dawley rats, using the following administration schemes:
- Acute toxicity study: 40 rats (20 from each sex); average weight: 90 g.
- Local tolerance study: 76 rats (38 from each sex); average weight: 90 g.
- Repeated-dose toxicity study: 110 rats (55 from each sex); average weight: 98 g.
The animals, supplied by the Center for the Production of Laboratory Animals (Cenpalab, Cuba), were clinically examined, weighed, housed in Makrolon cages with a bedding of bagasse, and kept under observation for 7 days under controlled environmental conditions (temperature 19-21 °C, average relative humidity 68 % and photoperiod of 12 h). The animals were fed daily with 25 g/individual of rodent chow (ALY co., Cenpalab, Cuba), quantifying daily food intake; water was supplied ad libitum. All experiments took place at Toxicology Block B of the Animal Experimentation Unit of CIGB (Havana, Cuba).
Test compound
The studies employed a formulation of the conjugate vaccine Quimi-Hib® adjuvanted with aluminum phosphate (CIGB, Havana, Cuba). Each vaccine vial contained 10 µg of conjugated PRP, 20.8 to 31.25 µg of tetanus toxoid, 0.05 mg/mL of thiomersal, q.s. and 0.25 mL of phosphate buffer. The vial of the adjuvant formulation contained 7.072 mg/mL of aluminum phosphate, 0.05 mg/mL of thiomersal and 0.25 mL of phosphate buffer, q.s. Analysis reports issued by the Quality Control Unit of CIGB confirmed the sterility, apyrogenicity, pH and general safety of this product. The results of the assays were satisfactory.
Experimental design
The studies were designed according to the regulations issued by the International Conference on Harmonisation (ICH) and the European Medicines Agency (EMA) [8-10]. Individual animals were randomly assigned to each experimental treatment with the help of a list generated by the Aleator software application (CIGB, Havana, Cuba; version 1.1, 1997). All doses were administered intramuscularly. Three dosages were evaluated, with the objective of providing a wide safety margin. The presence of a synthetic antigen was considered, and doses up to 45-fold the TD were examined. The latter corresponds to the maximum allowable volume according to the chosen administration route and host species, which constitutes the reference to establish the upper limit for dose and toxicity in the proposed experimental design. A satellite group receiving the highest dose in each case was included in the studies of local tolerance and repeated-dose toxicity, with the objective of evaluating the reversibility of any reported adverse events (Table 1).
All doses were calculated according to the body weight of the inoculated animals. Body weight ranged from 80 to 100 g at the beginning of the study. Six kilograms were used as the average weight of an 8-week old human infant. The TD recommended for future clinical trials was 10 µg. The test compound was inoculated into the femoral quadriceps of a hind limb [11].
Clinical examination, euthanasia and sampling
The animals underwent daily clinical examinations with the objective of detecting any behavioral variation or sign of toxicity such as changes in skin and fur, in eyes and mucous membranes or somatomotor activity. The following histological parameters were considered when evaluating local tolerance: presence of polymorphonuclear lymphocytes, local edema, acute hyperemia, hemorrhage and necrosis of muscle fibers. The animals were weighed weekly. Euthanasia was performed by cervical dislocation, after anesthetizing the animals in a CO2 chamber. All organs were examined under the microscope during necropsies, taking samples of liver, spleen, mesenteric lymph nodes, thymus and the inoculation site for later histopathological studies. During the repeated-dose toxicity study, the following hematological parameters were determined: eosinophil counts, hematocrit, hemoglobin concentration, mean corpuscular hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin concentration, and counts of leukocytes, platelets, neutrophils, lymphocytes, monocytes and eosinophils. Liver, spleen and thymus were also weighed.
Data processing
Statistical analyses were applied to body weight, average weekly food intake, hematological data, organ weight and histopathological findings, estimating measures of central tendency and dispersion (mean, standard deviation, maximum and minimum values). During the analyses of body weight and food intake, data normality and homogeneity of variance were examined for each evaluation time point and sex using the Shapiro-Wilk test with the Bonferroni correction for the interpretation of alpha error in paired tests and the Levene test, respectively, applying a parametric analysis of variance (ANOVA) or its non-parametric alternative (Kruskal-Wallis test) accordingly. Paired comparisons between consecutive time points were performed using a paired t-test or Wilcoxon’s test.
The histopathological data were analyzed using contingency tables with the associated test for independence (Fisher’s exact test).
RESULTS
Evaluation of acute toxicity
Body weight and food intake
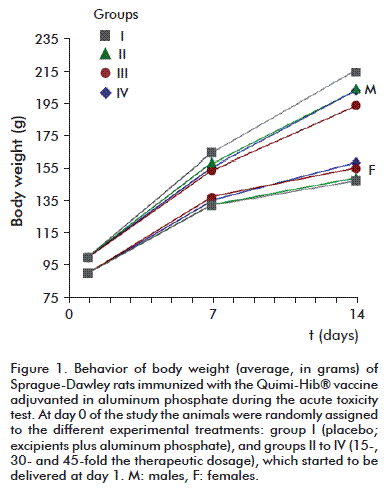
Body weight exhibited the expected behavior; that is, the values of this parameter between days 7 and 14 were significantly different and when compared to those of day 1 for each group, regardless of sex (Figure 1). On the other hand, no statistically significant differences were detected between treatment groups for the same time point (p > 0.05, Anova). Body weight increased at a rate significantly different from zero during the experimental stage of the study (p < 0.05, Anova).
Food intake was similar for all three studies, exhibiting a homogeneous behavior across groups for each evaluation time point: 15 to 18 g per day (Figure 2).
The highest food intake values were registered during weekends (days 5-6 and 12-13). This is an expected value, as during these days the stress arising from the manipulation of the animals is minimal. All environmental parameters remained stable, within the limits established for the species (rodents) [12-14]. No statistically significant inter-group differences were detected in the corresponding analysis (p > 0.05, Kruskal-Wallis test).
Clinical examination and macroscopic observations
No signs of toxicity or alterations were detected during the daily clinical examinations. Likewise, there were no deaths or behavioral alterations. No changes were detected in the inspected organs and tissues during necropsies.
Histopathological study
There were signs of inflammatory reactions in muscle tissue samples (hemorrhages or cicatrization foci), but there were no morphological alterations (Table 2).
The observed necrosis was not a lesion of the muscle tissue, but a central part of the granuloma. No statistically significant differences between placebo and vaccine-treated groups were detected. Group IV exhibited the least conspicuous alterations of the inoculation site (no symptoms in 6 out of 10 animals); an important finding, taking into account that these animals received 230 µL of the adjuvanted vaccine. There was extramedullary hematopoiesis in liver and spleen for all animals, a finding that was independent from immunogen type (vaccine vs. placebo) and dose. Likewise, there were no statistically significant differences between groups for the findings of the histopathological study. The examined organs (liver, spleen and mesenteric lymph nodes) did not exhibit alterations that might be construed as signs of toxicity.
Evaluation of local tolerance
Body weight
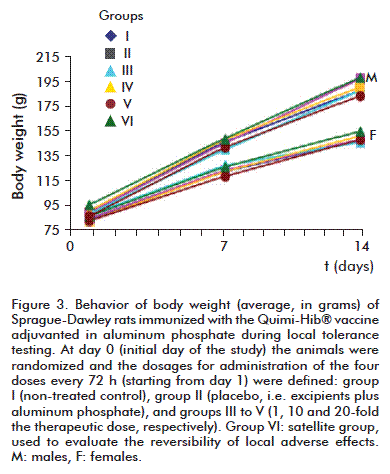
Body weight exhibited no statistically significant differences between experimental groups for the same time point (p > 0.05, Anova), and increased at normal rates (Figure 3). Average bodyweight increase was significantly different from zero during the evaluation period.
Clinical examinations and macroscopic observations
The most conspicuous systemic effect was the presence of marked piloerection in all animals, including those receiving the placebo, during week 2 of the study (after two immunizations). The only clinical sign detected during the study was induration at the inoculation site in groups II (placebo, i.e. excipients plus adjuvant), IV (medium dose), V (high dose) and VI (satellite group). No local reactions were detected during the first four days of the study, but mild indurations appeared, started from day 5, in the animals from groups II, V and VI except one individual from group VI, and remained present, with varying intensities, throughout the remainder of the study. Mild and moderate indurations were evidenced in the groups receiving 10 and 20-fold the TD and in the placebo group, which inoculation volume was the largest. Group VI (satellite) remained under observation up to day 28 of the study in order to evaluate the reversibility of these reactions. While they did not revert completely, the daily clinical reports evidenced a gradual decrease of their severity.
The macroscopic observations performed during necropsies (at 24 h, 7 and 14 days and 28 days for the satellite group) failed to find signs of damage in any organ system. There were white nodules at the inoculation site, which size was directly related to dose and administration volume.
Histopathological study
There was a noticeable increase in the number of macrophages at the inoculation site after 24 h of the first administration, which also exhibited liquid depots of the immunogen. These are typical and expected findings after the administration of an antigenic substance containing aluminum phosphate as an adjuvant, and were also observed during the necropsies performed at days 7, 14 and 28 although their intensity was variable, depending on dose number and size.
At day 7 (after 2 doses) the local reactions led to the appearance of diffuse macrophage granulomas with eventual liquid depots, a modest cicatrization response within the reaction and the infiltration of lymphocytes, especially in the periphery, with varying intensity. It should be noted that the granulomatose reaction of group III (TD) was also modest.
At day 14 (Table 3), after 4 doses, the inoculation site continued to exhibit the granulomas described above, which were most frequent and intense in the groups receiving the highest dose (V) and the largest volumes of adjuvant (II and V).
The only symptom of group I (non-treated control) was the presence of extramedullary hematopoiesis in the liver of two animals at day 7 and four animals at day 14, as well as extramedullary hematopoiesis in the spleen of five animals at days 7 and 14. No other histopathological signs were observed in this group during the study.
There were no cases of extramedullary hematopoiesis at the liver for any of the animals of the study at 24 h after the first inoculation. Likewise, there were no cases of alterations in lymph nodes or the thymus throughout the study. There was only a moderate inflammatory response and extramedullary hematopoiesis at the spleen of animals belonging to groups receiving adjuvant-containing formulations (including the adjuvant-only group) and extramedullary hematopoiesis in the liver of individuals from group III (TD).
There were evidences of granulation tissue only in two animals from group III (TD) at day 14.
Histopathological data for the satellite group was only collected at day 28. In this case, there was a modest local reaction in four animals, and a moderate reaction in another two; there was only one animal exhibiting extramedullary hematopoiesis. No other alterations were detected in this group.
The frequency of appearance of alterations in the other parameters is shown in table 3.
Evaluation of repeated-dose toxicity
Body weight and food intake
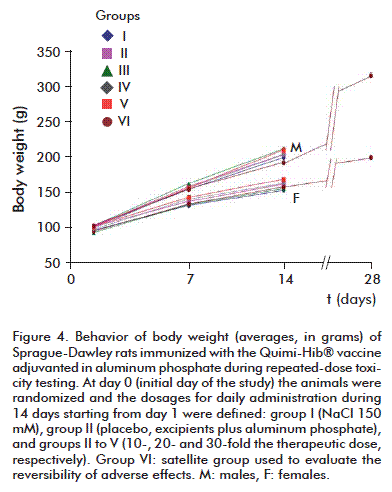
There were no differences regarding body weight between experimental groups for either sex at the same time point (p > 0.05, Anova), although average body weight did exhibit a statistically significant increase with respect to its initial value (p < 0.05, Anova) that was similar for every group (Figure 4).
Food intake also exhibited a homogenous behavior across groups for each time point (15 to 24 g per day) (Figure 5). Upon close examination, there was a significant decrease of average food intake by day 7 of the study for the females of group I, which was corroborated by the corresponding statistical analysis (p > 0.05, Kruskal-Wallis test).
Clinical examination and macroscopic observations
No signs of local reaction at the inoculation site or any other alterations were detected during the first 4 days after starting the study, although indurations at this site appeared afterwards in animals from every group and remained throughout the experimental stage. Severe induration and nodules were present in animals from the placebo, high dose (20 and 30-fold the TD) and satellite groups, which received the largest inoculation volumes, but were also observed in mild form in animals from group III (10-fold the TD).
The hematological parameters of treated groups were similar to those of animals from the same batch which did not receive any test compounds (control non-treated group). The macroscopic observations performed during necropsy confirmed the results of the clinical examinations; their main finding was the presence of pale formations with a nodular appearance. The weight of all organs remained within the normal ranges for the species.
Histopathological study
Muscle tissue samples were examined looking for histological signs of inflammatory reactions, hemorrhages and cicatrizing foci. However, our sole finding was the presence, in the animals from the groups receiving the largest vaccine doses, of diffuse granulomas in the inoculation site, circumscribed mainly to the endomysium of muscle fibers with liquid depots and evidence of cell lysis for the granulomatous tissue, interspersed with cicatrization tissue and a few polymorphonuclear eosinophils. The individuals from the placebo group did not exhibit granulomas, although three individuals did develop cicatrization tissue. None of the studied animals had evidence of liver damage, and all had histopathological evidence of antigenic stimulation on spleen and mesenteric lymph nodes. There were no differences regarding the magnitude and structure of the local reaction between the placebo group and the groups receiving multiple TD.
DISCUSSION
The sustained increase in body weight and stable food intake figures exhibited by all animals used for the preclinical safety testing of the aluminum phosphate-adjuvanted Quimi-Hib® conjugate vaccine evidence that this formulation is not toxic. Body weight increased consistently throughout the experimental stage up to the appointed date for euthanasia in all animals independently of the administered dose, resulting in statistically significant differences compared to the initial values (p < 0.05). The values of both body weight and food intake in every evaluation time point were similar to those reported for healthy animals of this species [15] even in groups receiving dosages as high as 45-fold the TD. These findings were confirmed by the clinical examinations, as there were no morphological or etiological alterations attributable to the test compound, evidencing the non-toxicity of the assayed formulations and treatments. In turn, the histopathological studies of each of the three experiments corroborated the findings of the clinical examinations. There was an inflammatory reaction at the inoculation site, which has been linked by several authors to the presence of aluminum phosphate as an adjuvant. This compound has been previously shown to efficiently potentiate effector inflammatory responses [16-18], characterized mainly by the presence of macrophages, neutrophils and plasma cells. The cicatrizing and hemorrhaging foci are simply a response to the mechanical trauma of the injections; as a matter of fact, there are occasional reports in literature of lacerations and damage to muscles and skin. In any case, most of the animals did not have alterations, further supporting the use of this formulation as a vaccine candidate. The histopathological study also pointed at aluminum phosphate as the main cause of the observed response, as our results coincide with those reported by other authors employing vaccines adjuvanted with aluminum salts [16-19]. The magnitude of the response corresponded to that expected after the repeated inoculation of this adjuvant for 14 days. The observed adverse effect at the inoculation site is not a sign of toxicity, despite the administration of an adjuvant concentration larger than that specified by the study protocol. These results evidence that the safety of the test compound, Quimi-Hib adjuvanted in aluminum phosphate, is similar to that of other vaccines approved for clinical use in many other countries [19-21].
There were no differences between placebo and three different TD dosages concerning the magnitude and structure of the local reaction after the repeated administration of the vaccine. This demonstrates that the detected histological reactions are mainly caused by the adjuvant and, especially, its concentration. Some authors have demonstrated the presence of aluminum at the center of the macrophage granulomas observed at the inoculation site after the administration of similar compounds [19], and others, such as Bordet [20], have reported the infiltration of neutrophils, microabscesses and the appearance of giant cells as markers of the adverse effect of aluminum salts-based adjuvants. However, these findings were not replicated by our study despite the use of adjuvant concentrations higher than those of other commercially available vaccines, demonstrating that the test compound satisfies all safety requirements for its examination in future clinical trials. The present investigation, in summary, did not find any effect attributable to the administration of the test compound that could be interpreted as a sign of potential toxicity.
The fact that the observed local signs reversed only partially coincides with previous observations by other authors (e.g. Gupta [17] and Goto et al. [22]), who reported that the indurations appearing at the inoculation site after the administration of vaccines adjuvanted with aluminum salts takes from 9 to 12 months to disappear both in rabbits and rats.
CONCLUSIONS
Taking into account the data from earlier studies with the Quimi-Hib vaccine and the results of the present work, it is concluded that the Quimi-Hib vaccine adjuvanted in aluminum phosphates meets all innocuity and safety requirements for its administration to human beings, as well as all national and international regulations for the use and marketing of this product type. The repeated administration of this product produces a local reaction characterized by macrophage granulomas of varying intensities. In addition, this product is not toxic at a systemic level, as its administration at a wide range of doses to Sprague-Dawley rats did not result in morphological alterations in any of the studied organs, did not alter their bodyweight increase rates and food intake indexes, did not alter any of the studied hematological parameters, and did not change the weight of the internal organs above or beneath the normal limits for healthy animals. The only relevant finding of the clinical examinations and the gross observations performed during necropsies was the presence of a local reaction at the administration site (indurations caused by macrophage granulomas), which has been previously reported for products employing aluminum salts as adjuvant and do not represent a sign of toxicity.
REFERENCES
1. Robbins JB, Schneerson R. Haemophilus influenzae type b: the search for a vaccine. Pediatr Infect Dis J. 1987;6(8):791-4.
2. Ahmed N, Gottschalk S. How to design effective vaccines: lessons from an old success story. Expert Rev Vaccines. 2009; 8(5):543-6.
3. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36-44.
4. Fitzwater SP, Watt JP, Levine OS, Santosham M. Haemophilus influenzae type b conjugate vaccines: considerations for vaccination schedules and implications for developing countries. Hum Vaccines. 2010;6(10):810-8.
5. Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13(2):302-17.
6. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055-65.
7. Verez-Bencomo V, Fernandez-Santana V, Hardy E, Toledo ME, Rodriguez MC, Heynngnezz L, et al. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science. 2004; 305(5683):522-5.
8. European Medicines Agency. CPMP/SWP/465/95. Note for guidance on preclinical pharmacological and toxicological testing of vaccines (CPMP adopted Dec 97). London: EMA; 1997 (cited 2012 Apr 16). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004004.pdf.
9. European Medicines Agency. CPMP/SWP/1042/99. Note for guidance on repeated dose toxicity (CPMP adopted July 2000). London: EMA. (cited 2012 Apr 16). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003102.pdf.
10. European Medicines Agency. CPMP/ICH/286/95. Note for guidance on Non-clinical safety studies for the conduct of Human clinical trials for pharmaceuticals. ICH M3 (R2). 2008. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002941.pdf.
11. Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21(1):15-23.
12. Cosme K. Programa para el Uso de Animales de Experimentación del Centro de Ingeniería Genética y Biotecnología. Edición 01;1998.
13. Van Zutphen LFM, Balls M . Animal Alternatives, Welfare and Ethics. Developments in Animal and Veterinary Sciences. Amsterdam: Elsevier Science Ltd.; 1997.
14. Olfert ED, Cross BM, Mc William A. Guide to the care and use of experimental animals. 2nd ed. Ontario: Canadian Council on Animal Care; 1998.
15. Harkness JE, Wagner JE. The Biology and Medicine of Rabbits and Rodents. 4th ed. Philadelphia, PA: Lea and Febiger; 1995.
16. Lindblad EB. Aluminium adjuvants-in retrospect and prospect. Vaccine. 2004; 22(27-28):3658-68.
17. Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32(3):155-72.
18. Harandi AM, Medaglini D, Shattock RJ. Vaccine adjuvants: a priority for vaccine research. Vaccine. 2010;28(12):2363-6.
19. Chong H, Brady K, Metze D, Calonje E. Persistent nodules at injection sites (aluminium granuloma) - clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48(2):182-8.
20. Bordet AL, Michenet P, Cohen C, Arbion F, Ekindi N, Bonneau C, et al. Granulome post-vaccinal lié à l’hydroxyde d’aluminium. Ann Pathol. 2001;21(2):149-52.
21. Makwana N, Riordan FA. Bacterial meningitis: the impact of vaccination. CNS Drugs. 2007;21(5):355-66.
22. Goto N, Kato H, Maeyama J, Shibano M, Saito T, Yamaguchi J, et al. Local tissue irritating effects and adjuvant activities of calcium phosphate and aluminium hydroxide with different physical properties. Vaccine. 1997;15(12-13):1364-71.
Received in May, 2012.
Accepted in January, 2013.
Dania Bacardí. Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11 600, La Habana, Cuba. E-mail: dania.bacardi@cigb.edu.cu.