Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.30 no.2 La Habana abr.-jun. 2013
REPORT
Transgenic plants of Nicotiana tabacum L. express aglycosylated monoclonal antibody with antitumor activity
Plantas transgénicas de Nicotiana tabacum L. expresan anticuerpo monoclonal aglicosilado con actividad antitumoral
Meilyn Rodríguez1, Merardo Pujol1, Lincidio Pérez1, Jorge V Gavilondo1, Greta Garrido2, Marta Ayala1, Marlene Pérez1, Mónica Bequet-Romero1, Gleysin Cabrera1, Osmani Ramos1, Ignacio Hernández3, Ernesto M González1, Vivian Huerta1, Belinda Sánchez2, Cristina Mateo2, Ada Triguero1, Osmani Mendoza1, Freya Freyre1, Carlos Borroto1
1 Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
2 Centro de Inmunología Molecular, CIM. AP 16040, CP 11300, Playa, La Habana, Cuba.
3 Centro de Isótopos, CENTIS. Ave. Monumental y Carretera “La Rada”, Km 31/2, Guanabacoa, La Habana, Cuba.
ABSTRACT
The expression and production of pharmaceutical, industrial and veterinary proteins in plants is an attractive approach. These expression hosts bear an enormous production potential in terms of volume, easy processing of the starting material, and safety of the final product due to the lack of pathogens able to infect animal and human cells. Antibodies are among the most frequently proteins expressed in plants, subsequently called plantibodies. However, plantibodies are differently glycosylated in plant cells, with oligosaccharide residues being added which may them immunogenic in the final organism. For that reason, several strategies have been developed to genetically modify host plants to mimic the N-glycosylation patterns typical in animal cells. This work was aimed at developing a strategy to obtain a aglycosylated plantibody version of nimotuzumab, the first antibody registered as a product in Cuba for cancer immunotherapy. The strategy comprised the genetic modification of the heavy chain glycosylation site of nimotuzumab, and its expression as an aglycosylated protein in tobacco leaves, by means of developing a transient expression system using Agrobacterium infiltration into tobacco leaves for the initial characterization of the plantibody. It was demonstrated that transgenic plants were capable of producing a plant-derived nimotuzumab antibody which retained the antitumor activity in vitro and in vivo, compared to its glycosylated counterpart produced in mammalian cells. This work demonstrates the potential of transgenic plants to produce aglycosylated therapeutic antibodies for cancer treatment, and won the National Award of the Academy of Sciences of Cuba in 2012.
Keywords: molecular farming, plantibody, aglycosylation, epidermal growth factor receptor, nimotuzumab.
RESUMEN
El empleo de plantas transgénicas para producir proteínas con fines farmacéuticos, industriales y ve-terinarios es una estrategia promisoria. Este sistema hospedero tiene un gran potencial productivo, en términos de volumen, fácil procesamiento y seguridad del producto final, por la ausencia de patógenos que infecten las células animales y humanas. Los anticuerpos están entre las proteínas más frecuentemente expresadas en las plantas, denominados planticuerpos. Sin embargo, los planticuerpos se glicosilan diferencialmente en las plantas pues se les adicionan residuos de oligosacáridos que pudieran hacerlos inmunogénicos en el organismo de destino. Con estos fines se han implementado estrategias para modificar genéticamente las plantas de forma que reproduzcan los patrones de N-glicosilación típicos de células animales. El propósito de este trabajo fue desarrollar una estrategia para obtener una versión aglicosilada del anticuerpo nimotuzumab, el primero registrado en Cuba como producto para la inmunoterapia del cáncer. Comprendió la modificación genética del sitio de N-glicosilación en la cadena pesada del anticuerpo y su expresión como proteína aglicosilada en hojas de tabaco. Para la caracterización inicial del planticuerpo se desarrolló un sistema de expresión transitoria por infiltración de Agrobacterium en hojas de tabaco. Las plantas transgénicas expresaron el planticuerpo PhR3 con similar actividad antitumoral que la mostrada por el nimotuzumab producido en células de mamíferos. Este trabajo demuestra la potencialidad del sistema de plantas transgénicas para producir anticuerpos terapéuticos aglicosilados para el tratamiento del cáncer, y mereció el Premio Nacional de la Academia de Ciencias de Cuba en 2012.
Palabras clave: agricultura molecular, planticuerpo, aglicosilación, receptor de factor de crecimiento epidérmico, nimotuzumab.
INTRODUCTION
The use of plants as bioreactors, or molecular farming, is a technology comprising both, the expression and characterization of recombinant proteins in plant hosts and its high scale production (host plant cultivation, harvesting and biomass storage, processing and purification of the protein of interest, and its related quality control processes and regulatory issues). The use of plants as bioreactors is a relatively new approach for biopharmaceutical production.
A comparison of plant expression systems to those based on other cell types shows that they have the same ability to do most of the posttranslational modifications required for the proper conformation of complex therapeutic proteins. Significantly, they can be scaled up to biomass production volumes unreachable for any other expression systems, and has the advantage of being generally-regarded-as-safe hosts, unable to bear pathogens capable of infecting animal and human cells. Noteworthy, it is possible to directly administer biopharmaceuticals by oral route as fruits, tubers, and others for therapy and vaccination [1]. Plants as expression systems are highly versatile, since they comprise various production platforms: whole plants, seeds, cells in suspension, roots, moss, algae and water duckweed. Furthermore, the expression can be targeted to different subcellular compartments or organs, by using specific molecular signals which facilitate better protection against posttranslational modifications, including proteolysis [1]. Among the most frequent plant species can be found: tobacco, tomato, banana, rice, corn, wheat, carrot, soybean, potato, lettuce and alfalfa [1]. Tobacco (Nicotiana tabacum L.) is the model by excellence because of its easy manipulation and genetic transformation, and its character of non-food crop which minimizes the risk of contamination of the food chain with recombinant proteins [2]. Particularly, tobacco leaves-based expression provides additional benefits, such as, higher biomass yields and minimal leakage of transformed genes into the environment due to the elimination of flowering; that is a shortcoming caused by pollen or seed dispersal.
Nevertheless, and as for any other eukaryotic expression systems, plants are unable to exactly reproduce human-type glycosylation patterns on biopharmaceuticals, taking into account that plant-specific glycosylation is considered at this stage as the major limitation for the use of plant-made pharmaceuticals in human therapy. To circumvent this disadvantage, many groups have been working on the humanization of protein N-glycosylation patterns by inactivating plant endogenous and/or expressing heterologous glycosyltransferases [3]. Another alternative comprises universal glycan structures obtained by targeting the recombinant protein to plant cell organelles with the aid of several signals (i.e., the KDEL tetrapeptide, to direct the expressed protein to the endoplasmic reticulum, ER) [4]. Instead of the proteins being mainly retained in those compartments, they also transit to other subcellular organelles such as Golgi cisterns [5], resulting in the addition of plant oligosaccharide structures (sugar moieties such as β-(1,2)-xylose and α-(1,3)-fucose). These processes may also vary, depending on the given plant species.
Antibodies are the most common type of recombinant proteins expressed in plants (so-called plantibodies) [4], intended for human therapeutic application in several human chronic diseases such as cancer, autoimmunity and persistent infections [6] and with real market perspectives. They are immunoglobulins which may require a very specific glycosylation pattern for its therapeutic action, conditioning its proper folding at the Fc region and for some subclasses of human IgG, particularly IgG1, the activation of complement system and antibody-dependent cell-mediated cytotoxicity (ADCC) [7]. At the same time, linked glycans may also affect the stability, immunogenicity and pharmacokinetic properties of these molecules. There is still insufficient information on whether glycosylated plantibodies can be effectively used for immunotherapeutics as their counterparts expressed in mammalian cells, to activate the complement system and promote ADCC. The same remains for the possible glycosylation-associated immunogenicity of the plantibody in humans, if it would be enough to interfere with the plantibody therapeutic action. Aglycosylation has also been investigated. Aglycosylated plantibodies have been obtained by mutating the N297 residue at the Fc region of the heavy chain, a strategy successful for plantibodies whose native antibody biological activity is independent of Fc region effector functions [8], incapable of interacting with its molecular target. As examples can be mentioned neutralizing antibodies, agonists or antagonists, or even when an active Fc fragment can produce unwanted side effects [8].
Taking into account all these aspects, a strategy was followed to obtain an aglycosylated plantibody variant of the humanized monoclonal antibody nimotuzumab (also known as hR3 or TheraCIM®), a genetically engineered product from the Center of Molecular Immunology (CIM, Havana, Cuba). Nimotuzumab is an isotype IgG1 antibody which targets the extracellular domain of the human epidermal growth factor receptor (EGFR) and disrupts the EGFR-associated signal transduction cascade and mitogenic effects [9]. Some authors have reported certain relationship between tumorigenicity and EGFR overproduction in a variety of human tumors, including lung cancer, astrocytic, head and neck tumors, among others [10]. The chemical-pharmaceutical characterization of nimotuzumab, together with preclinical information, production and testing in clinical trials in Cuba, allowed the registration of this product by the Cuban Regulatory Agency and the Center for State Control of the Quality of Medicines (Cecmed), as the first therapeutic antibody for the treatment of advanced head and neck in Cuba. It has also been used in several countries, in more than 10 therapeutic indications against several types of tumors such as: colorectal, pancreatic, prostate, esophageal and breast cancers [11].
The plantibody was obtained taking advantage of previous works from several groups at the Center for Genetic Engineering and Biotechnology (CIGB, Havana, Cuba), that established an efficient platform for the expression of heterologous proteins in plants [12]. The plantibody was expressed in transgenic Nicotiana tabacum L. as an aglycosylated variant, obtained by mutating the N-glycosylation site on the nimotuzumab heavy chain. The results supports the further therapeutic evaluation of the plantibody regardless the difference in glycosylation patterns depending on the expression host.
RESULTS AND DISCUSSION
Genetic construct for the expression in plants of an aglycosylated nimotuzumab antibody
The heavy and light chains of the humanized nimotuzumab antibody were amplified by polymerase chain reaction from the complementary DNA extracted from a murine transfectoma producing the antibody (donated by CIM). The design included suitable sites for insertion of genes into the plant expression vector (pHES74), and the plant endoplasmic reticulum sorting amino acid signal (KDEL) at the carboxyl terminus of both chains.
Additionally, the N297 amino acid was mutated to Q in the antibody heavy chain, eliminating the single glycosylation site of the immunoglobulin. Amplification products were sequenced, showing 98.2 % homology with the starting genes of the heavy and light chains of nimotuzumab. The pHES74-based constructs bearing each antibody chain were sequentially introduced into the binary vector pDE1001, resulting in the pD-EGFR plasmid carrying both transcriptional units in the same orientation. The tandem array of both expression cassettes into a single T-DNA would favor the coordinated expression of both genes. Thereafter, Agrobacterium tumefaciens was transformed with the plasmid of interest, for transient expression tests and stable transformation of plants.
Expression of the aglycosylated antibody in plant cells using a transient system
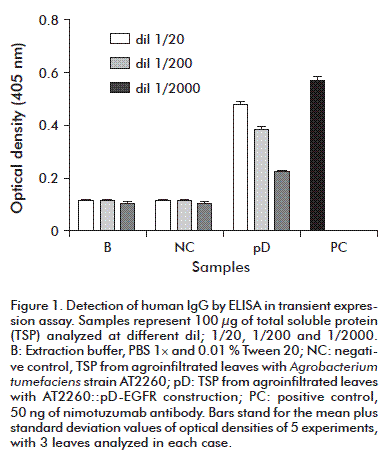
Our group developed a transient expression system based on vacuum infiltration of A. tumefaciens into the leaves of N. tabacum. This is a simple method by which a bacterium is deposited into the leaf tissues using a polycarbonate filter and, after infection, the leaves are used to detect the expression of genes of interest [13]. N. tabacum leaves infiltrated with A. tumefaciens suspensions were harvested at different days, while extraction of the total soluble proteins (TSP) was performed for evaluation. Plantibody expression was assessed by an ELISA detecting the presence of human IgG, as shown in figure 1, for leaves harvested on day 3 after infection.
Absorbance values obtained for the nimotuzumab antibody (positive control) are 5 times higher than the values used for the negative control of phosphate buffer saline (B; Figure 1). The values in the case of the TSP from leaves agroinfiltrated with the A. tumefaciens strain AT2260 (NC) were similar to those of B. These results indicated that the analytical system was specific for the detection of human IgG and did not react with other plant proteins. The optical density values of the TSP from leaves infiltrated with AT2260::pD-EGFR (pD) were approximately 4-fold those of the negative controls (Figure 1). These results indicated that the testing system detects the antibody produced in plant cells, and demonstrated the functionality of the pD-EGFR construct to express the aglycosylated variant of nimotuzumab.
The greater accumulation of the antibody detected on day 5 after infection could be due to the stability of the antibody within plant cells based on targeting to the ER, and also to the conditions and functionality of the tissue after agroinfection by the method developed in this study. This system enabled us to produce 1.2 µg of recombinant protein per gram of tissue, thereby facilitating downstream purification of the antibody by protein A affinity chromatography. The purified product was analyzed by SDS-PAGE and Western blot, demonstrating the absence of plants glycans. The biological activity was confirmed by an indirect immunofluorescence assay using the human tumor cell line A431 which over-expresses EGFR [13].
The implementation of agroinfection has advantages over other microbial systems for plant transformation, because of allowing the proper expression and postranslational processing of large heterologous complex proteins. Another advantage comprised the lack of sophisticated equipment and the low cost as compared to other transient systems such as microinjection, microprojectile bombardment or electroporation.
Production of aglycosylated plantibody from transgenic Nicotiana tabacum L. plants
Once the structural functionality and stability of the plantibody were corroborated by the transient expression system, transgenic plants were obtained as stable expression system able to generate progeny for production purposes, either by sexual (seeds) or asexual reproduction (by cuttings or micropropagation) [5].
Ninety-six transgenic plants were obtained by genetic transformation of N. tabacum variety Havana 2.1.1 leaves with recombinant A. tumefaciens AT2260::pD-EGFR. The presence of the antibody was demonstrated in 90 % of the clones, with clone 86 showing the highest accumulation of the plantibody (30 µg/g of fresh tissue). This plant line was propagated in vitro and subsequently grown under greenhouse conditions to obtain a homozygous line.
Leaves from 6-to-8-weeks old clone 86 transgenic plants were harvested and used as starting material to develop a protein A affinity chromatography purification process. A 50 % recovery was obtained at 5 kg scale, the plantibody preparation yielding 96 % purity as estimated by SDS-PAGE and gel densitometry. The product was named PhR3 and subjected to further analysis (Figure 2). Two bands of 25 and 50 kDa corresponding to the expected light and heavy chains of PhR3 were observed in lanes of the eluates processed under reducing conditions (Figure 2A). In the Western blot assay under non-reducing conditions, PhR3 plantibody appeared as a single band of high molecular weight (approximately 150 kDa), comparable to that of nimotuzumab (Figure 2B). The absence of oligosaccharides residues in the plantibody heavy chain was corroborated by enzymatic digestion with PNGase A followed by HPLC analysis [14].
Characterization of the immunochemical properties, biological and antitumor activity of the aglycosylated antibody
Purified PhR3 was characterized in vitro by using different techniques [14]. Its comparison to nimotuzumab showed that despite slight differences, the aglycosylation had no significant changes in the recognition of EGFR by ELISA, in living cells (flow cytometry assay), nor on the ability of plantibody to inhibit the phosphorylation and EGFR signaling. PhR3 was able to effectively displace radiolabeled EGF in a competition binding assay for EGFR in the microsomal fraction of human placenta. Also, the Fab fragment of PhR3 had a 6.1 × 10-8 M dissociation constant, an affinity parameter similar to that of nimotuzumab. In addition, PhR3 was also able to block cell cycle progression in human tumor cells in culture [14].
Moreover, similar results were found in the behavior of both molecules when looking at possible influences of aglycosylation in pharmacokinetics and biodistribution of PhR3, and its anti-tumor effect in vivo (Figure 3). Both molecules had similar pharmacokinetic profiles, characterized by a biphasic curve that could beadjusted to a two compartment model (Figure 3A). There was a statistically significant difference between both antibodies with respect to the distribution phase half-life (T½-α), indicating a larger and faster transfer to the peripheral compartment for the PhR3 plantibody. Noteworthy, no statistically significant differences were observed in the mean blood residence time (MRT) between both antibodies.
Figure 3B shows the results of an assay performed with different doses of both antibodies on nude mice bearing human tumor xenografts. No significant differences were found between groups of equal doses and treatments neither with PhR3 nor nimotuzumab.
CONCLUSIONS
In summary, this work demonstrates that transgenic plants can be successfully used to produce aglycosylated versions of immunoglobulins intended for therapeutic blocking of cell surface receptors or to prevent its interaction with the soluble ligands. Aglycosylated plantibody variants can be equally active as their mammalian counterparts, showing similar pharmacokinetics and biodistribution and at the same time exempt of plant-derived sugar moieties. Considering that nimotuzumab has received several approvals as therapeutic antibody for various specific types of cancer, the plant-derived nimotuzumab antibody could be a potential candidate for future cancer immunotherapy clinical developments.
RELEVANCE OF THE STUDY
The main contributions of this work were: i) the implementation of a strategy to express aglycosylated antibodies in transgenic leaves of tobacco plants, by using nimotuzumab as a model antibody; ii) the plant-derived nimotuzumab antibody showed biological properties similar to that of nimotuzumab, supporting its potential therapeutic use [14]; and iii) the transgenic tobacco plants were able to produce aglycosylated antibodies with the proper biological activity [14, 15]. Methodologically, the study also provided a new transient expression system to evaluate complex molecules in plants [13].
ACKNOWLEDGEMENTS
The authors thank the following collaborators for their contributions while conducting this study: Abel Hernández, Osvaldo Oliva, Pedro Luis Ramos, Jeny Soto, José Cremata† and Kenia Tiel from the CIGB; Rolando Pérez and Agustín Lage from CIM, and Mariela León and René Leyva from CENTIS.
REFERENCES
1. Xu J, Dolan MC, Medrano G, Cramer CL, Weathers PJ. Green factory: plants as bioproduction platforms for recombinant proteins. Biotechnol Adv. 2012;30(5):1171-84.
2. Morandini F, Avesani L, Bortesi L, Van Droogenbroeck B, De Wilde K, Arcalis E, et al. Non-food/feed seeds as biofactories for the high-yield production of recombinant pharmaceuticals. Plant Biotechnol J. 2011;9(8):911-21.
3. Borisjuk N, Komarnytsky S. Plant-derived antibodies for academic, industrial, and therapeutic applications. In: Pathak Y, Benita S, editors. Antibody-mediated drug delivery systems: Concepts, technology, and applications. Hoboken: John Wiley and Sons, Inc.. 2012. p. 365-82.
4. De Muynck B, Navarre C, Boutry M. Production of antibodies in plants: status after twenty years. Plant Biotechnol J. 2010;8(5):529-63.
5. Petruccelli S, Otegui MS, Lareu F, Tran Dinh O, Fitchette AC, Circosta A, et al. A KDEL-tagged monoclonal antibody is efficiently retained in the endoplasmic reticulum in leaves, but is both partially secreted and sorted to protein storage vacuoles in seeds. Plant Biotechnol J. 2006;4(5):511-27.
6. Elvin JG, Couston RG, van der Walle CF. Therapeutic antibodies: market considerations, disease targets and bioprocessing. Int J Pharm. 2013;440(1):83-98.
7. Jefferis R. Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol Sci. 2009;30(7):356-62.
8. Hristodorov D, Fischer R, Joerissen H, Muller-Tiemann B, Apeler H, Linden L. Generation and comparative characterization of glycosylated and aglycosylated human IgG1 antibodies. Mol Biotechnol. 2013;53(3):326-35.
9. Mateo C, Moreno E, Amour K, Lombardero J, Harris W, Perez R. Humanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activity. Immunotechnology. 1997;3(1):71-81.
10. Klein S, Levitzki A. Targeting the EGFR and the PKB pathway in cancer. Curr Opin Cell Biol. 2009;21(2):185-93.
11. Perez R, Moreno E, Garrido G, Crombet T. EGFR-Targeting as a Biological Therapy: Understanding Nimotuzumab’s Clinical Effects. Cancers. 2011;3(2):2014-31.
12. Pujol M, Ramirez NI, Ayala M, Gavilondo JV, Valdes R, Rodriguez M, et al. An integral approach towards a practical application for a plant-made monoclonal antibody in vaccine purification. Vaccine. 2005;23(15):1833-7.
13. Rodriguez M, Ramirez NI, Ayala M, Freyre F, Perez L, Triguero A, et al. Transient expression in tobacco leaves of an aglycosylated recombinant antibody against the epidermal growth factor receptor. Biotechnol Bioeng. 2005;89(2):188-94.
14. Rodriguez M, Perez L, Gavilondo JV, Garrido G, Bequet-Romero M, Hernandez I, et al. Comparative in vitro and experimental in vivo studies of the anti-epidermal growth factor receptor antibody nimotuzumab and its aglycosylated form produced in transgenic tobacco plants. Plant Biotechnol. J. 2013;11(1):53-65.
15. Pujol M, Gavilondo J, Ayala M, Rodriguez M, Gonzalez EM, Perez L. Fighting cancer with plant-expressed pharmaceuticals. Trends Biotechnol. 2007;25(10):455-9.
Meilyn Rodríguez. Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba. E-mail: meilyn.rodriguez@cigb.edu.cu.