Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.30 no.4 La Habana oct.-dic. 2013
RESEARCH
Cardiotropic effect of GHRP-6: in vivo characterization by echocardiography
Efecto cardiotrópico del GHRP-6: caracterización mediante ecocardiografía in vivo
Juan Valiente1, Diana García del Barco2, Gerardo Guillén2, Héctor Santana2, Fiorella Altruda3, Guido Tarone3, Lorenzo Silengo3, Jorge Berlanga2
1 Instituto de Cardiología y Cirugía Cardiovascular. Calle 17 e/ A y Paseo, No. 702, Vedado, Plaza de La Revolución, CP 10400, La Habana, Cuba.
2 Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
3 Centro de Biotecnología Molecular, Universidad de Torino, Italia.
ABSTRACT
A pharmacological therapy aimed at the multiple targets exposed by the complex pathophysiology of heart failure remains an unmet clinical need. One possible solution is the use of growth hormone secretagogue peptides, which have important cardiotropic properties. The aim of this work was to conduct an experimental evaluation of the effect of growth hormone releasing peptide six (GHRP-6) on heart function. Bi-dimensional experimental echocardiography was used to evaluate the cardiotropic effect of GHRP-6 when 400 µg/kg were administered to Balb/c mice, measuring the ventricular ejection fraction. Dose-dependency was studied with dosages of 100, 200 and 400 µg/kg, and the effect of concomitant beta-blocker usage on the effect of this peptide was assessed in animals chronically treated with metoprolol at 30 mg/kg. GHRP-6 increased the left ventricular ejection fraction without changes on heart rate. This inotropic effect was dose-dependent, and was sustained even in animals chronically treated with metoprolol.
Keywords: GHRP-6, inotropic effect, experimental bidimensional echocardiography, heart failure, myocardial ischemia-reperfusion, cardioprotection.
RESUMEN
Una terapia farmacológica que incida en múltiples blancos de la fisiopatología de la insuficiencia cardiaca es una necesidad clínica no satisfecha. Los péptidos secretagogos de la hormona de crecimiento se caracterizan por ejercer efectos cardiotrópicos relevantes. El objetivo de este trabajo fue evaluar, de manera experimental, el efecto del péptido 6 liberador de la hormona de crecimiento (GHRP-6) sobre la función cardiaca. Mediante la ecocardiografía bidimensional experimental, se evaluó el efecto cardiotrópico del GHRP-6 al administrar una dosis de 400 µg/kg en ratones Balb/c. Se estudió la respuesta en dependencia de la dosis: 100, 200 y 400 µg/kg, y el efecto sobre la función cardiaca en un grupo de animales que recibieron betabloqueo crónico con 30 mg/kg de metoprolol. El GHRP-6 incrementó la fracción de eyección ventricular izquierda, sin que aumentara la frecuencia cardiaca. El efecto inotrópico del GHRP-6 depende de la dosis y se mantiene, incluso, en los animales sometidos a betabloqueo crónico, sin que aumente la frecuencia cardiaca.
Palabras clave: GHRP-6, efecto inotrópico, ecocardiografía bidimensional experimental, insuficiencia cardiaca, isquemia-reperfusión miocárdica, cardioprotección.
INTRODUCTION
Cardiovascular disease remains the principal cause of mortality and morbidity in both developed countries and Cuba, reaching a toll of over 17 million new cases per year [1, 2]. Its most severe clinical manifestations, ischemic heart disease and myocardial infarction, are incapacitating disorders with high mortality rates despite the availability of therapeutic alternatives such as early reperfusion [3]. Even though mortality due to ischemic heart disease has diminished during the last 20 years, the incidence and prevalence of heart failure arising from this condition have grown dramatically, becoming the first cause of hospitalization and death among persons older than 65 years [4]. Ventricular remodeling, an adaptive repair process that attempts to correct myocardial damage, actually increases mortality and morbidity after myocardial infarction in high-risk populations [5].
The physiopathology of ventricular remodeling involves an intricate network of variations in gene expression together with molecular, cellular and interstitial modifications [6, 7] that all these produce changes in the size, shape and functional parameters of the damaged heart [5, 8]. Notwithstanding the complexity of this phenomenon, the range of therapeutic alternatives currently available is rather limited, essentially including the combination of pharmacological therapy (using beta-blockers, i.e. inhibitors of the Angiotensin converting enzyme and aldosterone blockers) [9-11], ventricular resynchronization therapy, regenerative therapy and heart transplant. To make matters worse, some of these alternatives are available only to a small group of patients.
Understandably, cardiologists have been looking for a single pharmacological therapy that can simultaneously hit many of the druggable targets exposed by the complex physiopathology of heart failure. From this point of view, natural or synthetic growth hormone secretagogues represent a potentially rewarding avenue of research, taking into account their beneficial effects on cardiovascular function. These include vasodilation and reduction of peripheral vascular resistance, increased microvascular flow, larger left ventricular ejection fraction, diminished diastolic dysfunction and inverse ventricular remodeling [12-14]. One of its most conspicuous examples, growth hormone releasing peptide 6 (GHRP-6), is a promising therapeutic candidate for post-infarction heart failure.
Previous results, obtained by evaluating GHRP-6 in experimental models of ischemia-reperfusion and dilated myocardiopathy [15, 16], confirmed many of these potential benefits. The present work intends, therefore, to evaluate echocardiographically whether the administration of GHRP-6 to experimental animals results in a positive inotropic response, and to examine the effect of this peptide on cardiovascular function when administered together with highly used cardiovascular drugs.
MATERIALS AND METHODS
Animals
The study employed male Balb/c mice weighing from 23 to 26 g, 8 to 10 weeks old (supplied by the Italian branch office of Charles River Laboratories). The animals were housed at a rodent room of the animal experimentation facility of the Center for Molecular Biotechnology of the University of Torino, Italy. Animal experimentation complied with all ethics and biosafety guidelines established by the institution. The mice were kept under controlled temperature, humidity and illumination, and received water and food ad libitum.
Peptide
Peptide GHRP-6 was synthesized at BCN Peptides S.A. laboratories (Barcelona, Spain). This studied employed its acetate salt, with batch number PC0601. The molecule was stored protected from light at -20 °C until diluted for administration to the animals, using physiological saline solution as the solvent. The experimental animals received a maximum dose of 400 μg/kg body weight, administered through the intraperitoneal route.
Anesthesia
The experiments were conducted under controlled conditions of volatile anesthesia, consisting on a mixture of isoflurane and medicinal oxygen delivered with a facial mask for rodents. No muscle relaxants or pre-anesthetic drugs were used. Doses used were 2.5 mL for anesthetic induction and 1.5 or 1 mL for maintaining a superficial anesthetic effect. The animals were attached, on their backs, to a platform whose position was controlled hydraulically, in order to guarantee a stable body temperature during experimentation and to ensure adequate mobility and proper placement of the transducer on the precordial zone. The transducer was, likewise, attached to a hydraulically controlled mechanical support arm.
Echocardiography
Bi-dimensional M-mode ultrasound images were obtained with a Vevo 770™ High-Resolution Imaging System (Visual Sonics Inc., Toronto, Ontario, Canada) fitted with a 40 MHz phased array linear transducer, acquiring data at frequencies up to 250 frames per second with an axial resolution of up to 30 microns in three focal zones. Data processing and analysis were performed with the Vevo 770 software application (version 3.0.0, Visual Sonics Inc.), on a view of the long parasternal axis at an angle of 45° and the caudal angulation of the platform (Figure 1).
Analytical parameters and methods
Measured bidimensional echocardiographic variables measured were: left ventricular end diastolic dimension (LVEDD), left ventricular end systolic dimension (LVESD), interventricular septal end diastolic dimension (IVSd), left ventricular end diastolic posterior wall dimension (LVPWd), fractional shortening (FS) and left ventricular ejection fraction (LVEF). This last parameter was calculated with Teichold’s equation:
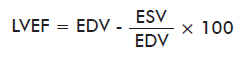
where: EDV, end-diastolic volume; ESV: end-systolic volume.
Measurements were taken according to the specific echocardiogram: diastolic parameters were measured at the peak of the R wave, and systolic parameters at the peak of the T wave. They were performed across 3 to 5 consecutive cardiac cycles, following the recommendations of the American Society of Echocardiography [17]. The determinations were performed at 5 min intervals, starting from minute zero before administering the peptide and then at 5, 10 and 15 min. In the case of the effect of beta-blockers, measurements continued to be performed up to 50 min post-administration.
Experimental design
The inotropic effect of GHRP-6 was evaluated in 18 mice, randomly assigned to either of two groups of 9 individuals each, receiving GHRP-6 or excipient respectively. Body weight, temperature and heart rate were measured, monitoring the echocardiogram during the experiment.
The dose-response relationship of the inotropic effect of GHRP-6 was evaluated with four experimental groups containing 8 individuals each. Group 1 was administered only the excipient, group 2 received 100 µg of GHRP-6 per kg of body weight, group 3 received 200 µg/kg of GHRP-6 and group 4 was administered the maximal dose of 400 µg/kg of GHRP-6. For every group, echocardiograms were obtained for time 0 or basal, and every 5 min afterwards until 15 min, under identical conditions of sedation, temperature and monitoring.
The effect of co-administering GHRP-6 and metoprolol (a beta-blocker) was determined using two groups of 8 mice, each receiving metoprolol-excipient or metoprolol-GHRP-6, respectively. In both cases, metoprolol (Seloken®, The Netherlands) [18] was first delivered subcutaneously in two daily doses of 30 mg/kg for a period of two weeks. At the end of this period, and coinciding with the last echocardiographic determinations, both groups were administered the corresponding metoprolol dose, and 15 min afterwards the metoprolol plus excipient group was administered the excipient via the intraperitoneal group, and the metoprolol plus GHRP-6 group received, in turn, 400 µg/kg of GHRP-6 using the same route. Echocardiographic determinations were performed before administering the last metoprolol dose, and later, every 5 min for a total of 50 min.
Statistical analysis
The data were analyzed using the statistical software package Prism (Graphpad Software Inc., San Diego, CA, USA). Means, medians and standard deviations were calculated as necessary. The dose-response effect was analyzed by linear regression. Differences were considered to be statistically significant for p < 0.05.
RESULTS
GHRP-6 has a positive inotropic effect but does not increase heart rate
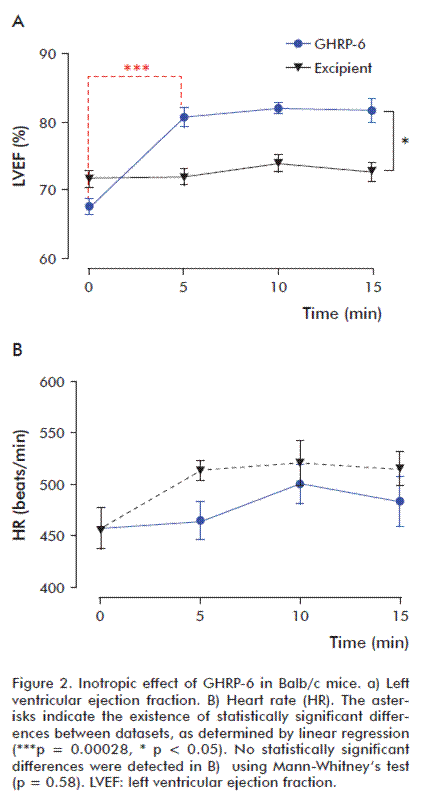
The systemic administration of peptide GHRP-6 produced a significant increase in the left ventricular ejection fraction (LVEF), which was not observed in the group treated with excipient (Figure 2A). This increase was most significant between 0 and 5 min (p = 0.0035), and remained statistically significant until the end of the observation period. LVEF increased on average by 15 % on the treated group. By contrast, in the control group treated only with excipient, LVEF increased only slightly, always under 5 % (a magnitude within the range of intra-assay variability). Heart rate remained 450 to 500 beats per min in both experimental groups (Figure 2B).
The inotropic effect of GHRP-6 exhibits dose-dependency
The administration of increasing doses of GHRP-6 produced a sustained and statistically significant increase of the left ventricular ejection fraction in the groups receiving 200 and 400 μg/kg GHRP-6 when compared to the group receiving only the excipient. The difference was not statistically significant in the case of the group receiving a dose of 100 μg/kg (Figure 3A). This result was confirmed by linear regression (Figure 3B).
During the first 10 min after the administration of different doses of GHRP-6, the positive inotropic effect of this compound exhibits a clear dose-response behavior (Figure 3C).
Inotropic effect of GHRP-6 under chronic beta-blocker use
The average basal ejection fraction in the animals treated with metoprolol was similar to that of animals in the untreated control group. After the administration of the day and before the echocardiographic determinations, there was a significant, 30 % decrease in systolic function for the left ventricle, which was sustained for the following 15 min. Heart rate also dipped below 300 beats/min. The injection of GHRP-6 produced a statistically significant increase in the ventricular ejection fraction at 5 min (p = 0.02), which progressed until LVEF returned to normal values and remained so to the end of the observation period (approximately 40 min). This effect was not observed in the control group that was administered only the excipient (Figure 4A). Heart rate remained well below the lower bound of the normal range for this species (Figure 4B) [19].
DISCUSSION
This is the first experimental bi-dimensional echocardiographic study demonstrating that GHRP-6 has a positive inotropic effect, as evidenced by the fact that its administration increases left ventricular ejection fraction. Bi-dimensional echocardiography is well suited to study the effect of this peptide on heart function, as it yields accurate, reproducible results with low intra-observer variability, affording the possibility of evaluating the heart as a whole.
It was demonstrated that the acute administration of peptide GHRP-6 to adult mice produced an early, significant increase in cardiac function (expressed as ejection fraction) that was sustained in time, without a concomitant increase in heart rate. The data also demonstrate that GHRP-6 can increase ventricular systolic function even in animals subjected to chronic treatment with a beta-blocker. The latter observation may hold significant value for the development of future therapeutic approaches to heart failure and ischemic cardiopathy in clinical settings, taking into account that a positive inotropic effect (i.e., favoring the contractility of heart muscle) and the absence of a chronotropic effect (i.e., no increase in heart rate) are desirable therapeutic outcomes. In other words, being able to increase myocardial function without a significant increase in oxygen consumption affords an important clinical advantage in the treatment of heart failure. To put it into perspective, it must be noted that all clinically available positive inotropes also increase heart rate through the stimulation of adrenergic receptors [20, 21] that, in turn, increase myocardial metabolic demand.
In clinical practice, it is possible to increase left ventricular function while simultaneously reducing heart rate and achieving vasodilation in patients with heart failure by using combinations of drugs such as amines, betablockers, inhibitors of the angiotensin converting enzyme and aldosterone [9-11]. Nevertheless, the finding that GHRP-6 is both a positive inotrope and a negative chronotrope is no doubt highly beneficial for current cardiologic practice.
The fact that GHRP-6 increases the ejection fraction in animals under chronic treatment with a beta-blocker suggests that its cardiotropic effect does not depend on the stimulation of cardiac beta-adrenergic receptors, unlike drugs such as sympathomimetic amines. In addition, this also indicates that the positive inotropic effect of GHRP-6 may not depend on the stimulation of this type of receptor; a novel finding that strongly supports its potential application for the therapy of systolic heart failure and its use as cardioprotector in myocardial ischemia-reperfusion events. Such an inference is supported by previous experimental evidence indicating that the cardiotropic effects of GHRP-6 are mediated by the stimulation of two different receptors, namely GHS-R1a and CD36 [22], as well as by recent data involving the latter, a multifunctional type B endocytic receptor, in the cardiotropic action of GHRP-6 [23].
Heart function in experimental models may also improve through a reduction of peripheral vascular resistance [24]. Since the present study was not aimed at detecting vasodilation, and taking into account that GHRP-6-like molecules have been previously shown to induce vasomimetic effects [25], we believe that focusing future research on evaluating whether GHRP-6 is in fact a vasodilator would be highly beneficial, as such a demonstration would no doubt increase the potential benefits of the application of this molecule to the therapy of heart failure.
CONCLUSIONS
It was demonstrated, using bi-dimensional experimental echocardiography, that GHRP-6 has a positive inotropic effect in mice. This effect is dose-dependent (100, 200 or 400 µg/kg), is detectable even under chronic beta-blocker usage, and is not associated to a positive chronotropic effect. Therefore, GHRP-6 can be considered as a potential therapeutic candidate for the treatment of severe heart failure and as a cardioprotector for myocardial ischemia-reperfusion events.
REFERENCES
1. Ministerio de Salud Pública, Cuba. Anuario estadístico de salud. La Habana: Dirección Nacional de Registros y Estadística de Salud; 2010 [cited 2013 Jul 7]. Available from: http://files.sld.cu/dne/files/2011/04/anuario-2010-e-sin-graficos1.pdf
2. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
3. Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359(5):473-81.
4. Salazar HP, Talano JV. Viable myocardium: how much is enough? Echocardiography. 2005;22(1):59-70.
5. Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008;1(5):582-91.
6. Hwang JJ, Dzau VJ, Liew CC. Genomics and the pathophysiology of heart failure. Curr Cardiol Rep. 2001;3(3):198-207.
7. Mirotsou M, Watanabe CM, Schultz PG, Pratt RE, Dzau VJ. Elucidating the molecular mechanism of cardiac remodeling using a comparative genomic approach. Physiol Genomics. 2003;15(2):115-26.
8. Pfeffer MA, Braunwald E. Ventricular enlargement following infarction is a modifiable process. Am J Cardiol. 1991;68(14):127D-31D.
9. Velazquez EJ, Pfeffer MA, McMurray JV, Maggioni AP, Rouleau JL, Van de Werf F, et al. VALsartan In Acute myocardial iNfarcTion (VALIANT) trial: baseline characteristics in context. Eur J Heart Fail. 2003;5(4):537-44.
10. Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893-906.
11. Flather MD, Yusuf S, Kober L, Pfeffer M, Hall A, Murray G, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355(9215):1575-81.
12. Benso A, Broglio F, Marafetti L, Lucatello B, Seardo MA, Granata R, et al. Ghrelin and synthetic growth hormone secretagogues are cardioactive molecules with identities and differences. Semin Vasc Med. 2004;4(2):107-14.
13. Cao JM, Ong H, Chen C. Effects of ghrelin and synthetic GH secretagogues on the cardiovascular system. Trends Endocrinol Metab. 2006;17(1):13-8.
14. Xu XB, Pang JJ, Cao JM, Ni C, Xu RK, Peng XZ, et al. GH-releasing peptides improve cardiac dysfunction and cachexia and suppress stress-related hormones and cardiomyocyte apoptosis in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;289(4):H1643-51.
15. Berlanga J, Cibrian D, Guevara L, Dominguez H, Alba JS, Seralena A, et al. Growth-hormone-releasing peptide 6 (GHRP-6) prevents oxidant cytotoxicity and reduces myocardial necrosis in a model of acute myocardial infarction. Clin Sci (Lond). 2007;112(4):241-50.
16. Cibrian D, Berlanga J, Guevara L, Valiente J, Guillen GE, Garcia D, et al. Efecto citoprotector cardiaco y extracardiaco del péptido GHRP-6. Biotecnol Apl. 2008;25(3):276-8.
17. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79-108.
18. Tominaga M, Matsumori A, Okada I, Yamada T, Kawai C. Beta-blocker treatment of dilated cardiomyopathy. Beneficial effect of carteolol in mice. Circulation. 1991;83(6):2021-8.
19. Matsuki N, Sakuma Y, Saito H. Pharmacological properties of blood pressure and heart rate control in suncus. Jpn J Pharmacol. 1993;62(1):93-7.
20. Tacon CL, McCaffrey J, Delaney A. Dobutamine for patients with severe heart failure: a systematic review and meta-analysis of randomised controlled trials. Intensive Care Med. 2012;38(3):359-67.
21. Morici N, Sacco A, Oliva F, Ferrari S, Paino R, Milazzo F, et al. Epinephrine for acute decompensated heart failure and low output state: friend or foe? Int J Cardiol. 2011;149(3):384-5.
22. Torsello A, Bresciani E, Rossoni G, Avallone R, Tulipano G, Cocchi D, et al. Ghrelin plays a minor role in the physiological control of cardiac function in the rat. Endocrinology. 2003;144(5):1787-92.
23. Sun Q, Zang WJ, Chen C. Growth hormone secretagogues reduce transient outward K+ current via phospholipase C/protein kinase C signaling pathway in rat ventricular myocytes. Endocrinology. 2010;151(3):1228-35.
24. Tivesten A, Bollano E, Caidahl K, Kujacic V, Sun XY, Hedner T, et al. The growth hormone secretagogue hexarelin improves cardiac function in rats after experimental myocardial infarction. Endocrinology. 2000;141(1):60-6.
25. Isgaard J, Granata R. Ghrelin in cardiovascular disease and atherogenesis. Mol Cell Endocrinol. 2011;340(1):59-64.
Received in February, 2013.
Accepted in July, 2013.
Juan Valiente. Instituto de Cardiología y Cirugía Cardiovascular. Calle 17 e/ A y Paseo, No. 702, Vedado, Plaza de La Revolución, CP 10400, La Habana, Cuba. E-mail: jvalient@infomed.sld.cu.