Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Biotecnología Aplicada
versão On-line ISSN 1027-2852
Biotecnol Apl vol.30 no.4 La Habana oct.-dez. 2013
REPORT
Identification of the first antagonist peptide that inhibits biological effects of interleukin-15
Identificación de la primera molécula peptídica que inhibe efectos biológicos de la interleucina-15
Alicia Santos1, Osvaldo Reyes1, Ania Cabrales1, Yunier Rodríguez1, Haydee Geronimo1, Hilda E Garay1, Celia A Arrieta1, Miriam Ojeda1, Ana C Machado1, José Suarez1, Julio A Ancisar1, Mariela Vázquez1, Gerardo Guillén1, Araceli Chico2, Miguel Estévez2, Alexey Llopiz1, Jesús Noda1, Aniel Sánchez1, Lorenzo Silengo3, Fiorella Artruda3, Silvio Perea1
1 Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
2 Hospital Clínico Quirúrgico Hermanos Ameijeiras. La Habana, Cuba.
3 University of Torino, Italy.
ABSTRACT
Interleukin-15 (IL-15) is a pro-inflammatory cytokine that is expressed in several autoimmune and inflammatory diseases. We have identified the 36-45 sequence KVTAMKCFLL on human IL-15 that is recognized by a soluble form of recombinant hIL-15Rα-Fc fusion protein. This sequence synthesized as a 10 aa. peptide binds to the IL-15Rαand was able to block the biological activity of IL-15 in two IL-15 dependent cells lines. Using alanine scan strategy we identified a more active peptide by replacing the second Lys in the sequence for the polar non-charged amino acid threonine. Moreover, soluble IL-15Rαwas quantitated by a newly developed enzyme-linked immunosorbent assay (ELISA) using the P8 peptide as capture in samples of synovial fluid from patients with rheumatoid arthritis (RA) and osteoarthritis (OA). This research won the 2012 Award of the Cuban National Academy of Sciences.
Keywords: antagonist, cytokine, IL-15, peptide, alpha receptor.
RESUMEN
La interleucina-15 (IL-15) es una citocina proinflamatoria que se expresa en varias enfermedades autoinmunes e inflamatorias. Se identificó la secuencia 36-45 KVTAMKCFLL de la IL-15 humana, reconocida por la proteína de fusión hIL-15Rα-Fc soluble. Esta secuencia, sintetizada como un péptido de 10 aminoácidos, se une a la IL-15Rαy bloquea la actividad biológica de la IL-15 en dos líneas celulares dependientes de IL-15. Mediante la estrategia de barrido de Ala, se detectó un péptido más activo por sustitución de la segunda lisina de la secuencia del péptido por el aminoácido polar, no cargado, treonina. Usando el péptido P8 en el paso de captura de un ensayo por inmunoabsorción ligado a enzimas (ELISA), recientemente desarrollado, se cuantificó IL-15Rα soluble en fluido sinovial de pacientes con artritis reumatoide (AR) u osteoartritis (OA). Este trabajo mereció el Premio Anual de la Academia de Ciencias de Cuba, en el año 2012.
Palabras clave: antagonista, citoquina, IL-15, péptido, receptor alfa.
INTRODUCTION
The development of biologic agents, that selectively block the effects of pro-inflammatory cytokines, has provided a major advance in the treatment of Rheumatoid arthritis (RA). This is a chronic autoimmune disease which affects 1 % of the population and is associated with significant morbidity and increased mortality. Imbalances in pro- and anti-inflammatory cytokines promote induction of autoimmunity, inflammation and joint destruction in RA [1]. Currently available TNF-α and IL-6 targeting biologic agents are highly effective. However, about 40 % of RA patients who receive a TNF inhibitor fail to achieve an adequate response and other patients discontinue TNF-blocking agents within the first year of treatment [2]. Therefore, other cytokines are being tested as targets in the clinic with promising results [3, 4].
IL-15 is a proinflammatory cytokine associated with several autoimmune diseases, particularly RA [5, 6]. Soluble IL-15 has been detected in synovia of patients with RA mainly expressed by macrophages, fibroblasts, and endothelial cells [7, 8], and there it recruits circulating memory T cells in the synovial membrane and may up regulate TNF-α, IL-17, and other pro-inflammatory cytokines [9-11]. Moreover, soluble IL-15 appears to be an important contributor to osteoclastogenesis contributing to bone erosion [12, 13]. Other two functional forms of IL-15 have been identified: IL-15R-independent membrane-bound IL-15 [14, 15] and membrane IL-15 anchored through IL-15Rα [16], although they have been less studied in RA.
Several studies have generated different IL-15 antagonists, such as neutralizing antibodies directed against IL-15 itself or alternatively, against IL-2R/IL-15Rα, mutant IL-15 molecules, and soluble fragments of the IL-15Rα chain linked to the immunoglobulin Fc element, that have been shown to be effective both in animal models and humans [17-19].
We focused our studies on finding an antagonist of IL-15/IL-15Rα binding because IL-15Rα is a specific IL15 subunit receptor that plays an important role in different mechanisms of action described for IL-15, and it has been shown that the IL-15/IL-15Rα complex is more active than IL-15 alone [20, 21]. We have identified a small peptide corresponding to the sequence 36-45 of IL-15 (KVTAMKCFLL) named P8, which specifically binds to IL-15Rα and exhibits an antagonist effect on IL-15 activity [22].
Moreover, we prepared a series of single points, Ala-substituted P8 peptide analogs to evaluate contribution of their individual amino acid side chains to IL-15Rα binding. As a result, we have identified the peptide [K6T]P8 exhibiting a ten-fold enhanced antagonist activity. Both wild type P8 and this more active analog [K6T]P8 inhibit secretion of TNF-α, a validated target in RA [23]. Finally, we used P8 peptide in immunoassays to determine the presence of soluble IL-15Rα in synovial fluid and its potential role in inducing reverse signaling through membrane-bound IL-15 on cells from synovial fluid. Interestingly, we found higher levels of IL-15Rα in RA compared with osteoarthritis (OA), and also we found that there is a positive relationship between these high levels of IL-15Rα and high levels of IL-6 in RA but not in OA [24].
RESULTS
Identification of a binding sequence to IL-15Rα
The peptide spot synthesis approach was used to identify regions of IL-15 involved in the binding to IL-15Rα. The cellulose sheets displays 22 peptides that comprise entire human IL-15 sequence were incubated with IL-15Rα fused to Fc of human IgG1. We observed a strong signal on spot 8 corresponding to the 36-45 sequence KVTAMKCFLL on mature IL-15 [22]. No positive spots were observed when cellulose was incubated with human antibody IgG1, ruling out the possibility that IL-15Rα-Fc occurred on binding through its Fc region.
P8 competitively inhibits the binding of IL-15 to IL-15Rα and IL-15 biological activity
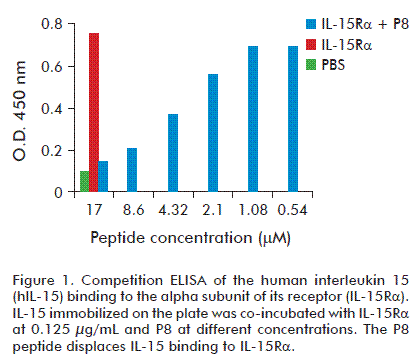
Competitive ELISA was performed to test the binding specificity of the P8 peptide to IL-15Rα. The sequence corresponding to spot 8 (KVTAMKCFLL) was synthesized as a soluble linear 10 aa. peptide. IL-15Rα-Fc and different concentrations of P8 peptide were co-incubated with IL-15 immobilized in the plate and the bound IL-15Rα-Fc was detected with HRP-conjugated goat anti-human IgG or with an antibody anti IL-15Rα development in goat; and latter incubated with HRP-conjugated mouse anti-goat IgG. Both immunoassays showed similar results. As shown in figure 1, the P8 peptide displaces the binding of IL-15Rα to hIL-15 in a dose-dependent manner.
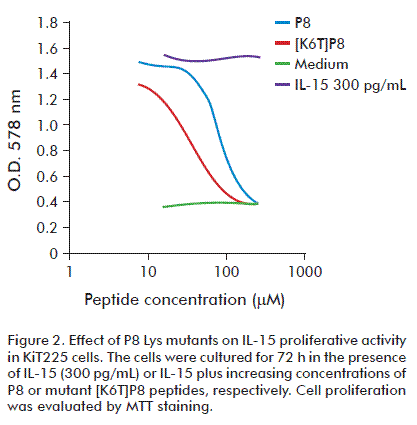
Biological activity of P8 peptide was tested for its ability to inhibit IL-15 activation of cell proliferation of two IL-15 dependent cell lines: the murine CTLL-2 cell line and the human KiT225 cell line that expresses the trimeric receptor IL-15Rαβγ. The P8 peptide inhibited IL-15-induced proliferation in a dose-dependent manner at plateau concentrations of 300 pg/mL of IL-15 in the KiT225 human leukemia cell line, showing a 50 % inhibitory concentration (IC50) of 130 µM (Figure 2). The inhibitory effect of the P8 peptide was also dependent on IL-15 concentration in the CTLL-2 cell line and the P8 peptide alone was unable to affect the proliferation of these cells induced by IL-2 [23].
Therefore, with the aim to study the contribution of each amino acid to the antagonist effect of P8 peptide and reduce its IC50 (130 μM), we synthesized a family of single Ala mutants of this peptide. Then, they were evaluated by preincubation with IL-15Rα, and subsequently, the resulting mixtures were added onto IL-15-coated surfaces in order to measure the ability of each synthetic peptide to competitively inhibit IL-15/IL-15Rα complex formation. We found that Phe and Cys are important for peptide binding to IL-15Rα. Other single point mutations were investigated and the second Lys in the sequence was replaced by the polar non-charged amino acid threonine. Interestingly, we found that the replacement of Lys41 by Thr generated the peptide [K6T]P8 of higher antagonist activity than P8 in CTLL-2 cell proliferation assays, showing an IC50 value of 24 μM [23].
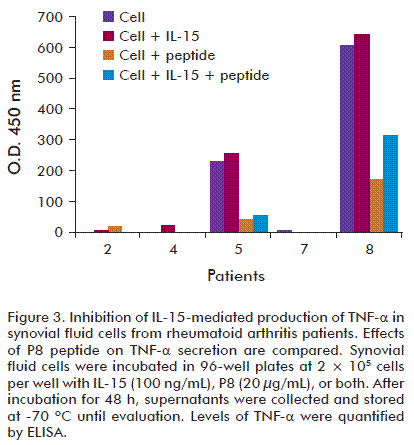
In order to assess activity of P8 and its analog [K6T]P8 on other IL-15-induced biological effects, we measured the effect of these peptides on TNF-α secretion. Synovial cells from RA patients with high levels of IL-15 in synovial fluids were incubated with P8 and the mutant [K6T]P8 in the presence of IL-15 for 72 h, and then, TNF-α levels were determined by ELISA. We found that both peptides inhibited TNF-α secretion from a pool of synovial cells and, in agreement with previous results obtained for IL-15-dependent cell lines, [K6T]P8 was more inhibitory on TNF-α secretion than the P8 peptide (Figure 3).
Usefulness of P8 peptide as a tool for detecting IL-15Rα
We used the P8 peptide as capture to measure the IL-15Rα level in synovial fluid from patients with RA (n = 18) or OA (n = 17) using our previously developed ELISA [16]. Soluble IL-15Rα (sIL-15Rα) was detected in 100 % of RA patients (18/18) and in 82.3 % of OA patients (14/17). That was the first report on detecting sIL-15Rα in synovial fluid. A significant increase in concentrations of sIL-15Rα was observed in synovial fluid collected from RA patients compared to those from OA patients (Figure 1 in reference [24]).
RELEVANCE OF THE STUDY
The first antagonist peptide described for IL-15 which specifically binds to IL-15Rα subunit and inhibits cell proliferation and proinflammatory cytokine secretion induced by IL-15 was obtained in this work. The strategy described summarizes the identification, assessing of its biological activities and its capacity as a tool to detect IL-15Rα. Therefore, this peptide is a potential drug as blocker of IL-15 in diseases such as RA, and can be used as immunoreagent to detect IL-15Rα in biological fluids. These results have two patents granted.
ACKNOWLEDGEMENTS
We thank the following collaborators of the CIGB for their contribution to this work: Karelia Cosme, Noraylis Lorenzo, Dagmara Pichardo, Sonia González and Yordanka Masforrol.
REFERENCES
1. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429-42.
2. Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther. 2009;11 Suppl 1:S1.
3. Maini RN, Taylor PC. Anti-cytokine therapy for rheumatoid arthritis. Annu Rev Med. 2000;51:207-29.
4. McInnes IB, Liew FY. Cytokine networks-towards new therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005;1(1):31-9.
5. McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2(2):175-82.
6. Carroll HP, Paunovic V, Gadina M. Signalling, inflammation and arthritis: Crossed signals: the role of interleukin-15 and -18 in autoimmunity. Rheumatology (Oxford). 2008;47(9):1269-77.
7. McInnes IB, Gracie JA, Harnett M, Harnett W, Liew FY. New strategies to control inflammatory synovitis: interleukin 15 and beyond. Ann Rheum Dis. 2003;62 Suppl 2:ii51-4.
8. Oppenheimer-Marks N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells In vitro and in the SCID mouse-human rheumatoid arthritis model In vivo. J Clin Invest. 1998;101(6):1261-72.
9. McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3(2):189-95.
10. Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170(4):2106-12.
11. Verri WA Jr, Cunha TM, Ferreira SH, Wei X, Leung BP, Fraser A, et al. IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol. 2007;37(12):3373-80.
12. Ogata Y, Kukita A, Kukita T, Komine M, Miyahara A, Miyazaki S, et al. A novel role of IL-15 in the development of osteoclasts: inability to replace its activity with IL-2. J Immunol. 1999;162(5):2754-60.
13. Miranda-Carus ME, Benito-Miguel M, Balsa A, Cobo-Ibanez T, Perez de Ayala C, Pascual-Salcedo D, et al. Peripheral blood T lymphocytes from patients with early rheumatoid arthritis express RANKL and interleukin-15 on the cell surface and promote osteoclastogenesis in autologous monocytes. Arthritis Rheum. 2006;54(4):1151-64.
14. Budagian V, Bulanova E, Orinska Z, Pohl T, Borden EC, Silverman R, et al. Reverse signaling through membrane-bound interleukin-15. J Biol Chem. 2004;279(40):42192-201.
15. Neely GG, Epelman S, Ma LL, Colarusso P, Howlett CJ, Amankwah EK, et al. Monocyte surface-bound IL-15 can function as an activating receptor and participate in reverse signaling.J Immunol. 2004;172(7):4225-34.
16. Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17(5):537-47.
17. Baslund B, Tvede N, Danneskiold-Samsoe B, Petersen J, Petersen LJ, Schuurman J, et al. A novel human monoclonal antibody against IL-15 (humax-IL15) in patients with active rheumatoid arthritis (RA): results of a double-blind, placebocontrolled phase I/II trial. Arthritis Rheum. 2003;48 Suppl 9:S1706.
18. Kim YS, Maslinski W, Zheng XX, Stevens AC, Li XC, Tesch GH, et al. Targeting the IL-15 receptor with an antagonist IL-15 mutant/Fc gamma2a protein blocks delayed-type hypersensitivity. J Immunol. 1998;160(12):5742-8.
19. Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160(11):5654-60.
20. Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Ralpha. Proc Natl Acad Sci USA. 2006;103(24):9166-71.
21. Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J Biol Chem. 2006;281(3):1612-9.
22. Santos A, Cabrales A, Reyes O, Gerónimo H, Rodríguez Y, Garay H, Arrieta C, Silva R, Guillen G. Identification of an interleukin-15 antagonist peptide that binds to IL-15Rα. Biotecnol Apl. 2008;25(4):320-4.
23. Savio AS, Acosta OR, Perez HG, Alvarez YR, Chico A, Ojeda MO, et al. Enhancement of the inhibitory effect of an IL-15 antagonist peptide by alanine scanning. J Pept Sci. 2012;18(1):25-9.
24. Machado Diaz AC, Chico Capote A, Arrieta Aguero CA, Rodriguez Alvarez Y, Garcia Del Barco Herrera D, Estevez Del Toro M, et al. Proinflammatory soluble interleukin-15 receptor alpha is increased in rheumatoid arthritis. Arthritis. 2012;2012:943156.
Alicia Santos. Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba. E-mail: alicia.santos@cigb.edu.cu.