Meu SciELO
Serviços Personalizados
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Biotecnología Aplicada
versão On-line ISSN 1027-2852
Biotecnol Apl vol.31 no.3 La Habana jul.-set. 2014
RESEARCH
Elucidation of the effects of inoculum size and age on lipase production by Geotrichum candidum
Determinación del efecto del tamaño y la edad del inóculo en la producción de lipasa por Geotrichum candidum
Rafael Resende Maldonado1, Janaína Fernandes Medeiros Burkert2, Elizama Aguiar-Oliveira3, Lúcia Durrant4, Márcio A Mazutti5, Francisco Maugeri Filho6, Maria I Rodrigues6
1 Food Department, Technical College of Campinas, University of Campinas, Campinas-SP, Brazil.
2 Chemistry Engineering Department, Federal University of Rio Grande, Rio Grande-RS, Brazil.
3 Multidisciplinary Institute on Health, Federal University of Bahia, Vitória da Conquista-BA, Brazil.
4 Food Science Department, Food Engineering Faculty, University of Campinas, Campinas-SP, Brazil.
5 Chemistry Engineering Department, Federal University of Santa Maria, Santa Maria-RS, Brazil.
6 Food Engineering Department, Food Engineering Faculty, University of Campinas, Campinas-SP, Brazil.
ABSTRACT
Lipases are extremely versatile enzymes with large industrial applications and they have been the subject of investigation over the last few years. A great variety of microorganisms can produce this enzyme, such as pluricellular fungi, but their growth need to be optimized due to the variations amongst experiments, which influence the results and reproducibility of the process. The aim of this work was to study the best conditions for inoculum size and age of Geotrichum candidum NRRLY-552, to reduce the variability of the inoculum. The optimized inoculum procedure was determined as: 1 circular area (0.79 cm2) of the solid medium containing cells and spores added to 100 mL of culture medium (5.0 % w/v of peptone, 0.1 % w/v of NaNO3, 0.1 % w/v of MgSO4 and 1.0 % w/v of soybean oil) incubated for 15 h at 30 °C, 250 rpm and with an initial pH of 7.0. This procedure permitted to reduce the experimental error from 30 to 20 % and it has been applied successfully in different studies since then.
Keywords: lipase, solid inoculum, soybean oil, factorial design, Geotrichum candidum.
RESUMEN
Las lipasas son enzimas extremadamente versátiles. Se utilizan en varias aplicaciones industriales, y han sido objeto de atención recientemente. Una diversidad de microrganismos puede producirlas, tales como los hongos pluricelulares. Sin embargo, para el estudio de estas enzimas se debe optimizar su crecimiento, debido a las variaciones entre los experimentos, que influyen en los resultados y en la reproducibilidad de los procesos. El objetivo de este trabajo fue investigar el tamaño y la edad idóneos del inóculo Geotrichum candidum NRRLY-552, con el fin de reducir su variabilidad. El procedimiento optimizado para el inóculo se determinó como una zona circular (0.79 cm2) de medio sólido, que contenía células y esporas, añadido a 100 mL de medio de cultivo (peptona 5.0 % p/v, NaNO3 0.1 % p/v, MgSO4 0.1 % p/v y aceite de soya 1.0 % p/v) que se incubaron durante 15 h a 30 °C, 250 rpm, con un pH inicial de 7.0. Este procedimiento permitió reducir el error experimental de 30 a 20 %, y se ha aplicado con éxito en varios estudios.
Palabras clave: lipasa, inóculo sólido, aceite de soya, diseño factorial, Geotrichum candidum.
INTRODUCTION
Lipases (triacylglycerol acyl hydrolases, E.C. 3.1.1.3) are enzymes with considerable physiological significance and industrial potential [1-3]. They have several applications in organic chemistry and pharmaceutical processes, detergent formulations, biosurfactant synthesis, oleochemical industry, dairy and agrochemical industries, paper manufacture, nutrition, cosmetics, and more recently in biofuel production, among other uses [4-9]. Due to their wide-range significance, lipases remain the subject of intensive studies focused particularly on their structural characterization, elucidation of action mechanisms, kinetics, sequencing and cloning of lipase genes and general performance characterization.
Additionally, the development and management of the microorganism inoculum through various production stages have a definite effect on the subsequent performance of the process. In commercial industrial fermentation processes, it is well known that the age and density of the inoculum used directly influences on the duration of the lag phase, specific growth rate, biomass yield, sporulation and quality of the final product, and hence on production costs [10,11]. Therefore, few researchers have investigated these variables in lipases and other enzymes production [12-14].
Previously, substrate concentration, pH and inoculum size were found to be the most important factors for lipase production by Penicillium cyclopium by using response surface methodology [15, 16]. A strong interaction was also detected between the primary and secondary inoculums on a two-stage inoculum system [17]. Moreover, the inoculum size was an important factor in an experimental design to obtain high enzymatic activity [18]; this variable was also relevant in the production of an alkaline protease by Bacillus mojavensis [19].
Recent studies on the production, efficiency and application of lipases from Geotrichum sp. and G. candidum NRRL-Y 552 [20-24], remarked the importance of inoculum variables such as size, volume and age for lipase production by Geotrichum sp. through an optimized experimental design. Significantly, the best result of 35 U/mL was obtained with 0.78 cm2 of inoculum size, 50 mL of inoculum volume medium and 12 h of inoculum age using corn steep liquor and soybean oil in fermentation medium [25].
Additionally, it is very common to obtain fungal spores for fermentation using a spore solution, but that methodology is very imprecise.
Therefore, the aim of this work was to develop an optimized inoculum procedure for lipase production by Geotrichum candidum NRRLY-552, including the use of a solid circular area (SCA) containing spores as an inoculum alternative methodology. This procedure allowed to reduce the inoculum variability under the tested conditions and to improve standardization, reliability and reproducibility parameters of lipase activity results. The inoculums size and age were investigated and a fractional factorial design was used to show the impact of experimental modifications on lipase production.
MATERIALS AND METHODS
Inoculum procedures
Geotrichum candidum NRRLY-552, an imperfect fungus, was provided by the Agricultural Research Service Collection. It was cultivated in Yeast Malt Extract Agar (YMEA) slants (0.3 % (w/v) malt extract, 0.3 % (w/v) yeast extract, 0.5 % (w/v) peptone, 1.0 % (w/v) glucose and 3.0 % (w/v) agar) for 48 h at 30 ºC, and stored at 4-5°C until use [24]. The inoculum medium was composed of (w/v): 5.0 % peptone, 0.1 % NaNO3, 0.1 % MgSO4 and 1.0 % soybean oil, with an initial pH = 7.0; the fungus was incubated for 24 h at 30 °C and 250 rpm.
The traditional procedure for inoculum preparation consists of transferring one loopful of cells and spores from the YMEA slants to 10 mL of inoculum medium. After incubation, the total volume was transferred to an erlenmeyer of 500 mL containing 100 mL of the same inoculum medium and incubated at the same conditions.
A different procedure was studied for the optimization of inoculum concerning the size and age. First, from the YMEA slants, spores and cells were suspended in 1 mL of sterile distilled water by scraping the surface with a loop, this volume was then poured on a Petri dish containing YMEA and spread with a Drigalski spatula; incubation proceeded for 48 h and at 30 °C. After this incubation, the surface of the agar medium on the Petri dishes were uniformly covered by the mycelium and its spores, supporting the assumption that a fixed inoculum size will always provide a very similar concentration of cells, and consequently, spores. From these YMEA Petri dishes, solid circular areas (SCA) were cut off by pressing the edge of a sterilized test tube with a specific diameter (ø) on the agar medium covered with cells and spores; the SCA were then removed carefully and transferred to an erlenmeyer of 500 mL containing 100 mL of inoculum medium volume (IMV).
Effect of the inoculum size
The effect of inoculum size was investigated in the production of lipase by G. candidum NRRLY-552. Fermentation medium composition and incubation conditions were the same as applied for the inoculum, except for the fermentation time which was 72 h.
Firstly, from 1 to 4, solid circular areas (SCA) of 1.54 cm2 (ø = 1.4 cm) were used to prepare the inoculum, then, the fermentation medium was inoculated at 10 % (v/v) using the resulting inoculum. The lipase activity and pH were determined during fermentation and the best result was considered for the next test.
In sequence, two variables were studied: SCA of 0.79 and 1.54 cm2, and IMV from 50 to 300 mL to determine the best inoculum size. The lipase activity and pH were determined during the incubation time, and the best conditions selected to make a third test to define the inoculum size.
Based on the results obtained at the first and second tests, four new fermentations were finally conducted using SCA of 0.79 and 1.54 cm2, and IMV of 50 and 100 mL at the same fermentation conditions. The lipase activity and pH were determined during the fermentation time.
Effect of inoculum age
The effect of inoculum size was investigated in the production of lipase by G. candidum NRRLY-552. Fermentation medium composition and incubation conditions were the same as applied for the inoculum, except for the fermentation time which was 72 h.
The inoculum was prepared using one SCA of 0.79 cm2 (ø = 1.0 cm) and 100 mL of inoculum medium. The incubation time of the inoculum varied from 15 to 48 h and the lipase activity and pH were determined as response during the fermentation time.
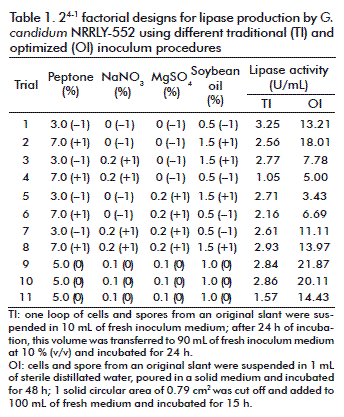
After the determination of the optimum inoculum conditions (inoculum size and age), a 24-1 experimental design with 3 central points was conducted for two different inoculums procedures: traditional and optimized. For the first factorial design, the traditional inoculum was applied as described above; for the second factorial design, the optimized inoculum was obtained with 1 solid circular area (SCA) of 0.79 cm2, 100 mL of inoculum medium incubated for 15 h. The independent variables studied were the concentrations (w/v) of: peptone (3.0-7.0 %), MgSO4 (0-0.2 %), NaNO3 (0-0.2 %) and soybean oil (0.5-1.5 %) in the composition of the fermentation medium; the response analyzed was the lipase activity (U/mL). Fermentation proceeded for 48 h at 30 °C and 250 rpm. The matrix, with coded and real values for the independent variables and the response values obtained, is presented in table 1; the results were analyzed using the software Statistica 7.0 Statsoft Inc.
In order to analyze the reproducibility of the process, four new trials with the optimized inoculum were carried out under the optimum conditions (central points; w/v): 5 % peptone, 0.1 % MgSO4, 0.1 % NaNO3, and 1.0 % soybean oil; initial pH of 7.0. Fermentation was carried out at 30 °C and 250 rpm. Lipase activity and pH were measured during fermentation time.
Lipase assay
Lipase activity was measured using a titrimetric assay, titrating with 0.05 M NaOH and using emulsified olive oil as substrate. The reaction mixture consisted of 19 mL of an olive oil/Arabic gum emulsion (5 % w/v olive oil and 5% w/v Arabic gum) in 100 mM potassium phosphate buffer, pH 7.0. This mixture was homogenized in a blender for 3 min and the enzyme reaction started by adding 1 mL of culture supernatant. The assay was carried out at 37 ºC and 200 rpm for 30 min. The reaction was then stopped by adding 20 mL of acetone-ethanol 1:1 (v/v), and the amount of fatty acids produced titrated with 0.05 M NaOH to pH 11.0 using an automatic titration apparatus (Mettler DL21). One unit of lipase activity (U) was defined as the amount of enzyme that releases 1 μmol of fatty acid per minute under the assay conditions [22, 24, 26].
RESULTS AND DISCUSSION
Effect of inoculum age
Initially, four experiments were carried out to evaluate the influence of the inoculum size on lipase production. In the first experiment one SCA with 1.54 cm2 (ø = 1.4 cm) was added to the inoculums medium, while in the second, third and fourth experiments were added 2, 3 and 4 SCA with 1.54 cm2, respectively. There was a decrease in lipase activity with an increase in inoculum size (number of SCA) during the fermentation time (Figure 1A). The highest activity (15.8 U/mL) was obtained with 1 SCA after 48 h, a value about 6 times higher than with 4 CSA, suggesting that a smaller number of spores added to the same substrate volume is better to obtain higher levels of lipase activity during fermentation.
This fact can be related to a typical phenomenon found in mycology: the self-inhibition of fungal spore germination. Many fungal spores exhibit a crowding effect [27-29], in which the spores contain a prepackaged self-inhibitor that prevents germination under crowded conditions. Another effect observed was that fungi with small inoculum sizes produced a transient mycelial stage with the mycelium length inversely proportional to the inoculum size [30]. This effect was also obtained in the production of cellulase by Trichoderma reesei Rut C-30, in which the average dimension of the pellet seemed to be inversely proportional to the inoculum size [31].
Based on these first results, two different values of SCA were chosen: 1.54 cm2 (ø = 1.4 cm) and 0.79 cm2 (ø = 1.0 cm), since the first analysis suggested that the smaller the inoculum size, the more efficient the process. In addition, different IMV from 50 to 300 mL were also evaluated, their lipase activities shown in table 2; the pH data are not presented. The highest activities were obtained with a SCA of 0.79 cm2 and an IVM of 50 mL (trial 1) and 100 mL (trial 2), were 7.53 and 7.74 U/mL obtained after 24 and 48 h, respectively. These results reinforce the hypothesis that the smaller quantity of spores in the inoculums, the greater lipase activity obtained. It was previously observed that Geotrichum sp. inoculum size and volume had a negative effect on lipase production [25].
Finally, based on the best results obtained so far, the effect of inoculum size was evaluated using inoculum conditions of SCA 0.79 and 1.54 cm2 (ø = 1.0 and 1.4 cm) and IMV of 50 and 100 mL. Lipase activities obtained are presented in figure 2. The highest lipase activity of 13.20 U/mL was obtained at 37 h in trial 2: SCA 0.79 cm2 and IMV 100 mL. For comparison, the optimized inoculum defined for Geotrichum sp. consists of the same SCA 0.79 cm2 but IMV 50 mL [25].
Spore cultivation on a solid medium is rarely mentioned in the literature, but in practical fermentation studies, this technique considerably reduces variations in the quantity of spores used to make the inoculums, and the results obtained were reproducible as compared with those obtained using spore measurement. There was reported the production of xylanase by Pleurotus ostreatus SYJ042 following the same methodology as used in the present work, i.e., spore cultivation on a solid medium and the transference of SCA to the inoculum medium; the same SCA (0.79 cm2) was determined using four 0.5 cm diameter disks (ø). The authors stated that smaller SCA and larger volumes were ideal for inoculum preparation [32]. In another study, it was shown with soil bacteria that the decreased inoculum size resulted in significant increases in the viable counts, assessed as colony forming units on solid media [12].
The pH analysis for all the tests (data not shown), revealed that pH values dropped to around 6.0-6.5 during the first 24 h of fermentation and increased almost to 8.0 at the end, when the lipase activity starts to decrease. That high pH value is not favorable to lipase production profiles, as demonstrated in Figure 1b, an effect commonly observed with G. candidum [22, 24].
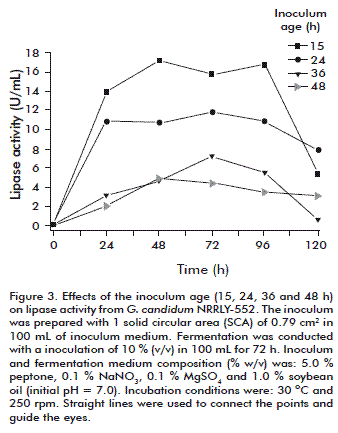
Inoculum age
The influence of the inoculum age was analyzed using the best conditions determined for the inoculum size: 1 SCA, 0.79 cm2, and IMV 100 mL. As shown in figure 3, a 15-h inoculum showed the best results in terms of lipase activity levels, around 16.5 U/mL from 48 to 96 h. The age of inoculum is highly variable depending on the process, cultivation conditions, medium composition and the microorganism, among other factors. The best condition for the inoculum age of G. candidum determined in this work was shorter than in previous studies using different microorganisms. In fact, it was optimal because of reducing the total fermentation time and increasing productivity. Inoculum age values of 18 and 96 h were previously obtained during alkaline protease production by Bacillus mojavensis [19] and Bacillus sp. [33], respectively. Nevertheless, considering lipase production from Geotrichum sp. [25], the inoculum age was set as 12 h, a result quite similar to that obtained in this study with G. candidum. The pH data are not shown but followed the same profile as described earlier (Figure 1 b).
Comparison of lipase production using different inoculums procedures
It is known that factorial designs can also be used to compare the results obtained with modifications not directly included in the independent variables analyzed [34]. Therefore, two 24-1 factorial designs were applied to investigate the variability of lipase production with the optimized inoculums, and also with the traditional inoculum procedure (Table 1). The aim was to investigate the variability of the experiments with different inoculum procedure, not to compare both conditions in relation to their lipase activities. For that reason, the central points conditions were the same already been applied for G. candidum cultivation (inoculum and fermentation medium).
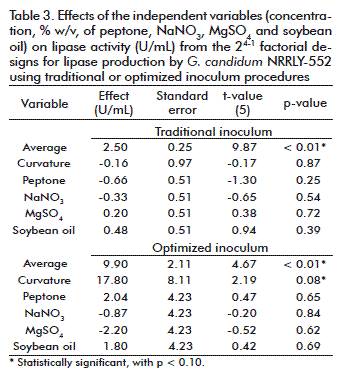
Considering the traditional inoculum (Table 1), the central points (trials 9, 10 and 11) resulted in an average activity of 2.42 ± 0.74 U/mL and the variables conditions (trials 1 to 8) resulted in an average activity of 2.51 ± 0.67 U/mL, respectively. The deviation observed at the central points corresponded to 30.58 % of the main value, a high deviation that must be avoided. The analysis of the effect (Table 3) showed that with the traditional inoculum, the different conditions in medium composition did not influence lipase activity and were not statistically significant, which can be seen clearly from the average activities and its deviation mentioned above and in the average activity and its deviation (2.50 ± 0.25 U/mL) considering all experiments (trials 1 to 11). This suggests that there were problems in respect to the standardization and reproducibility of the inoculum conditions, which did not respond well to the variation in medium composition because of its own variation. That effect was not observed with the optimized inoculum.
According to the results (Table 1) with the optimized inoculum, at the central points conditions (trials 9, 10 and 11) the lipase activities resulted in an average value of 18.80 ± 3.89 U/mL and at the variables conditions (trials 1 to 8) the average activity was 9.90 ± 5.01 U/mL. Considering all experiments (trial 1 to 11) the average activity was 9.90 ± 2.11 (Table 3). These values reflect the variability of results: at the same conditions, the central points presented a deviation of 20.69 % from the main value. Therefore, a well-defined inoculum avoids an extra variability to the study and allows more confidence in the responses obtained. At first, the analysis of the effects of the independent variables (Table 3) showed that with the optimized inoculum all the variables were not statistically significant in the range studied (p < 0.10). But after modifying the inoculum curvature it became statistically significant, indicating that the central point conditions were better for lipase production. This is in agreement with the fact that this conditions are already been used for inoculum and fermentation medium composition.
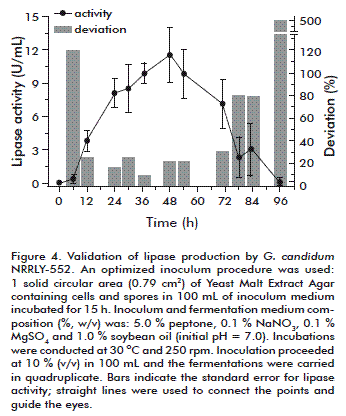
The reproducibility of the results was assessed with four new trials using the optimized inoculum at the same conditions of the central points; the results are presented in figure 4. The highest level of lipase activity was achieved between 24 and 54 h; the error in relation to the average values was around 20 %, which is acceptable for fermentation using filamentous fungi. After 48 h, lipase activity was 11.51 ± 2.52 U/mL and its deviation 21.91 % of the main value. In a previous study, using an optimized inoculum procedure and an optimized medium composition but with Geotrichum sp., the maximum lipase activity obtained was 35.2 ± 0.8 U/mL, its deviation corresponding to 2.27 % of the main value [25].
At this point, it is important to state that the optimized inoculum procedure here presented was lately applied for the optimization of medium composition for G. candidum cultivated in peptone [24], achieving a maximum lipase activity of 16.3 ± 0.8 U/mL with a deviation of 5 % of the main value. Recently, the inoculum procedure was also applied to the optimization of yeast hydrolysate and corn steep liquor instead of peptone in medium composition, and the maximum lipase activities obtained were 18.4 ± 0.8 and 21.7 ± 2.4 U/mL after 48 h, respectively [35, 36]. Deviations were equivalent to 4.3 and 9.5 % of the main value, respectively. From these results is possible to observe that the optimization of the inoculum procedure in this study was valid.
The pH data are not presented but it behaved as expected, with a slow decrease around 24 h followed by an increase until reaching 8.0 after 54 h, further increased until 8.8 after 96 h of fermentation. As previously discussed, the pH value of 8.0 is detrimental for lipase activity as can be seen in figure 4, with lipase activity showing a drop starting after 54 h to almost 1.0 U/mL at 96 h.
CONCLUSIONS
The conditions determined in this study with respect to inoculum size and age contributed to improve lipase production by G. candidum NRRL Y-552 and to reduce the variability on fermentation results. From this study it was possible to obtain a maximum lipase activity of 11.51 ± 2.52 U/mL after 48 h with an optimized inoculum. This result is valid since it was possible to obtain smaller deviations (~20 %) than those obtained with the traditional inoculum (~30 %), which support much more reliable studies, especially about other important factors.
The inoculum strategy presented here has been applied successfully in lipase production by G. candidum, resulting in 16.3 ± 0.8 U/mL (48 h) in shaken flasks [24], 21 ± 0.7 U/mL (54 h) in a bench-scale stirred reactor and 20 U/mL (30 h) in an airlift bioreactor [21]. It was also used for lipase production by Geotrichum sp. in shaken flasks, achieving 35.20 ± 0.80 U/mL [25], and to improve it by Fusarium oxysporum with a 60 % increase [2]. The errors observed by those authors were around 5 % of the main values of activities, which represent a great achievement in fungi cultivation.
ACKNOWLEDGEMENTS
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for their financial support.
REFERENCES
1. Alberghina L, Schmid RD, Verger R, editors. Lipases: structure, mechanism and genetic engineering, Weinheim: VCH; 1991.
2. Maldonado RR, Macedo GA, Rodrigues MI. Lipase production using microorganisms from different agro-industrial by products. Int J App Scien Tech. 2014;4(1):108-15.
3. Treichel H, de Oliveira D, Mazutti MA, Di Luccio M, Oliveira JV. A review on microbial lipases production. Food Bioprocess Technol. 2010;3(2):182-96.
4. Aravindan R, Anbumathi P, Viruthagiri T. Lipase applications in food industry. Ind J Biotech. 2007;6:141-58.
5. Gandhi NN. Applications of lipase. J Amer Oil Chem Soc. 1997;74(6):621-34.
6. Gopinath SCB, Anbu P, Lakshmipriya T, Hilda A. Strategies to Characterize Fungal Lipases for Applications in Medicine and Dairy Industry. BioMed Res Int. 2013;Article ID 154549. [cited 2014 Dic 16]. Available from: http://dx.doi.org/10.1155/2013/154549.
7. Ribeiro BD, de Castro AM, Coelho MA, Freire DM. Production and use of lipases in bioenergy: a review from the feedstocks to biodiesel production. Enzyme Res. 2011;2011:615803.
8. Sharma R, Chisti Y, Banerjee UC. Production, purification, characterization, and applications of lipases. Biotechnol Adv. 2001;19(8):627-62.
9. Tan T, Lu J, Nie K, Deng L, Wang F. Biodiesel production with immobilized lipase: A review. Biotechnol Adv. 2010;28(5):628-34.
10. Monhaghan MM, Gagliardi SL, Streicher AL, Demian JE. Culture preservation and inoculum development. In: Demain AL, Davies JE, editors. Manual of Industrial Microbiology and Biotechnology. 2nd ed. Washington: ASM Press; 1999.
11. Stanburry PF, Whitatker J, Hall SJ. Principles of Fermentation Technology. Oxford: Pergamon, 1995.
12. Davis KE, Joseph SJ, Janssen PH. Effects of growth medium, inoculum size, and incubation time on culturability and isolation of soil bacteria. Appl Environ Microbiol. 2005;71(2):826-34.
13. Kumar R, Mahajan S, Kumar A, Singh D. Identification of variables and value optimization for optimum lipase production by Bacillus pumilus RK31 using statistical methodology. Nat Biotechnol. 2011;28(1):65-71.
14. Teng Y, Xu Y. Culture condition improvement for whole-cell lipase production in submerged fermentation by Rhizopus chinensis using statistical method. Bioresour Technol. 2008;99(9):3900-7.
15. Vanot G, Deyris V, Guilhem MC, Phan Tan Luu R, Comeau LC. Optimal design for the maximization of Penicillium cyclopium lipase production. Appl Microbiol Biotechnol. 2001;57(3):342-5.
16. Vanot G, Valerie D, Guilhem MC, Phan Tan Luu R, Comeau LC. Maximizing production of Penicillium cyclopium partial acylglycerol lipase. Appl Microbiol Biotechnol. 2002;60(4):417-9.
17. Sen R, Swaminathan T. Response surface modeling and optimization to elucidate and analyze the effects of inoculum age and size on surfactin production. Biochem Eng J. 2004;21(2):141-8.
18. Kammoun R, Naili B, Bejar S. Application of a statistical design to the optimization of parameters and culture medium for alpha-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by-product). Bioresour Technol. 2008;99(13):5602-9.
19. Beg QK, Sahai V, Gupta R. Statistical media optimization and alkaline protease production from Bacillus mojavensis in a bioreactor. Process Biochem. 2003;39(2):203-9.
20. Asses N, Ayed L, Bouallagui H, Ben Rejeb I, Gargouri M, Hamdi M. Use of Geotrichum candidum for olive mill wastewater treatment in submerged and static culture. Bioresour Technol. 2009;100(7):2182-8.
21. Burkert JF, Maugeri F, Rodrigues MI. Optimization of extracellular lipase production by Geotrichum sp. using factorial design. Bioresour Technol. 2004;91(1):77-84.
22. Burket JFM, Maldonado RR, Maugeri F, Rodrigues MI. Comparison of lipase production by Geotrichum candidumin stirred and airlift fermenters. J Chem Tech Biotechnol. 2005;80(1):61-7.
23. Kamimura ES, Mendieta O, Sato HH, Pastore G, Maugeri F. Production of Lipase from Geotrichum sp. and Adsorption Studies on Affinity Resin. Braz J Chem Eng. 1999;16(2):102-12.
24. Maldonado RR, Panciera AL, Macedo GA, Mazutti MA, Maugeri F, Rodrigues MI. Improvement of lipase production from Geotrichum sp. in shaken flasks. Chem Ind Chem Eng Quart. 2012;18(3):459-64.
25. Maldonado RR, Burkert JFM, Mazutti MA, Maugeri F, Rodrigues MI. Evaluation of lipase production by Geotrichum candidum in shaken flasks and bench-scale stirred bioreactor using different impellers. Biocat Agric Biotechnol. 2012;1(2):147-51.
26. Freire DM, Teles EM, Bon EP, Sant’ Anna GL, Jr. Lipase production by Penicillium restrictum in a bench-scale fermenter : effect of carbon and nitrogen nutrition, agitation, and aeration. Appl Biochem Biotechnol. 1997;63-65:409-21.
27. Aver’yanov AA, Lapikova VP, Pasechnik TD, Zakharenkova TS, Baker CJ. Self-inhibition of spore germination via reactive oxygen in the fungus Cladosporium cucumerinum, causal agent of cucurbit scab. Eur J Plant Path. 2011;130(4):541-50.
28. Griffin DH. Fungal physiology. 2nd ed. New York:Willey-Liss; 1994.
29. Macko V, Staples RC, Yaniv Z, Granados RR. Self-inhibitors of fungal spores germination - The fungal spore. New York: Academic Press; 1976.
30. Kulkarni RK, Nickerson KW. Nutritional control of dimorphism in Ceratocystis ulmi. Exp Mycol. 1981;5(2):148-52.
31. Domingues FC, Queiroz JA, Cabral JM, Fonseca LP. The influence of culture conditions on mycelial structure and cellulase production by Trichoderma reesei Rut C-30. Enzyme Microb Technol. 2000;26(5-6):394-401.
32. Qinnghe C, Xiaoyu Y, Tiangui N, Cheng J, Qiugang M. The screening of culture condition and properties of xylanase by white-rot fungus Pleurotus ostreatus. Proc Bioch. 2004;39(11):1561-6.
33. Genckal H, Tari C. Alkaline protease production from alkalophilic Bacillus sp. isolated from natural habitats. Enz Microb Technol. 2006; 39(4):703-10.
34. Rodrigues MI, Iemma AF. Planejamento de Experimentos e Otimização de Processos. 2nd ed. Campinas: Caritas; 2009.
35. Maldonado RR, Aguiar-Oliveira E, Pozza EL, Costa FAA, Maugeri Filho F, Rodrigues MI. Production of lipase from Geotrichum candidum using corn steep liquor in different bioreactors. J Amer Oil Chem Soc. 2014; 91(12):1999-2009.
36. Maldonado RR, Aguiar-Oliveira E, Pozza EL, Costa FAA, Mazutti MA, Maugeri F, et al. Application of yeast hydrolysate in extracellular lipase production by Geotrichum candidum in shaken flasks, stirred tank and airlift reactors. Can J Chem Eng. Accepted in November, 2014.
Received in June 2014.
Accepted in December 2014.
Rafael Resende Maldonado. Food Department, Technical College of Campinas, University of Campinas, Campinas-SP, Brazil. E-mail: ratafta@yahoo.com.br.