My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.31 no.3 La Habana July.-Sept. 2014
TECHNIQUE
Implementation of a competitive ELISA for pharmacokinetics studies of CIGB-300 in human plasma
Implementación de un ELISA de competencia para estudios de farmacocinética del péptido CIGB-300 en plasma humano
Vilcy Reyes1, Yasser Perera1, Euyení Díaz1, Ileana Rosales2, Gerardo García2, Silvio E Perea1
1 Laboratorio de Oncología Molecular, Subdirección de Investigaciones Biomédicas, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
2 Departamento Control de la Calidad, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
ABSTRACT
CIGB-300 is a synthetic peptide that inhibits the phosphorylation mediated by enzyme casein kinase 2 (CK2) and has a marked antineoplastic effect in different preclinical models. In the clinical setting, it’s explored in phase I and II studies using different routes of administration. In particular, the use of the intravenous route requires a reliable analytical method for the detection of CIGB-300 in plasma. A competitive ELISA was developed to detect and quantify the CIGB-300 peptide in human plasma samples. This system showed a detection limit of 0.030 µg/mL and a working range from 10 to 0.039 µg/mL, including concentrations achieved in plasma of patients treated with CIGB-300. In addition, the intra and inter-assay precisions were (coefficient of variation < 5 %) and (CV < 17 %) and the recovered range from 98.9 to 119.8 %. Finally, the impact of three freeze-thaw cycles and the sample storage at - 80 °C on the stability of the analyte was evaluated. We obtained a CV < 20 % for all samples in the stability study. The results support the application of this analytical method as a new tool for the pharmacokinetic studies of the early stages of clinical research with the new anticancer drug CIGB-300.
Keywords: CIGB-300, ELISA, system validation, pharmacokinetics, intravenous route, anticancer drug.
RESUMEN
El CIGB-300 es un péptido sintético que inhibe la fosforilación mediada por la enzima caseína quinasa 2 (CK2) y ejerce un marcado efecto antineoplásico en diferentes modelos preclínicos. En estudios fase I y II, se exploran sus efectos por varias vías de administración. En particular, la vía intravenosa requiere un método analítico confiable para su detección en plasma. Con este propósito, se desarrolló un ELISA de competencia, para detectar y cuantificar el péptido CIGB-300 en muestras de plasma humano. Este sistema mostró un límite de detección de 0.030 µg/mL y un rango de trabajo de 10 a 0.039 µg/mL, que incluye las concentraciones alcanzadas en el plasma de pacientes tratados con CIGB-300. Además se determinó la variabilidad intraensayo (CV < 5 %) e interensayo (CV < 17 %) y el rango de recobrado del sistema (de 98.9 a 119.8 %). Finalmente, se evaluó el impacto de hasta tres ciclos de congelación-descongelación y de la conservación de la muestra a -80 °C sobre la estabilidad del analito. Para todas las muestras del estudio de estabilidad se obtuvo un coeficiente de variación menor del 20 %. Los resultados fundamentan la aplicación de este método analítico como una nueva herramienta para los estudios farmacocinéticos de las primeras etapas de la investigación clínica con el nuevo fármaco anticáncer CIGB-300.
Palabras clave: CIGB-300, ELISA, validación, farmacocinética, vía intravenosa, fármaco anticáncer.
INTRODUCTION
CIGB-300 is a synthetic peptide inhibiting the phosphorylation mediated by the casein kinase 2 (CK2) enzyme and exerting a marked anti-neoplastic effect in different in vitro and in vivo preclinical models [1]. Experimental evidence in cell lines derived from lung, cervix, prostate and colon cancer suggest that the multifunctional protein B23/NPM, a validated substrate of CK2, is a relevant molecular target for CIGB-300 in tumor cells [2]. As the result of extensive in vivo pharmacological experimentation, data were collected on antitumor efficacy and safety that are the pre-clinical basis for the studies of the CIGB-300 peptide in clinical trials [3].
The CIGB-300 peptide was first tested in human beings in the year 2006. This pioneering clinical study consisted of its direct application in pre-malignant cervix lesions and gave the first robust evidence of the safety of the product [4]. Nonetheless, two of the oncological niches now under study are the malignant neoplasia of the lung and acute myeloid leukemia (AML). These locations must be treated with the systemic administration of the drug since their intervention is difficult (as is lung cancer) or it is a disseminated disease (as in AML). In this context, the implementation of an analytical system, enabling the estimation of the concentration of the compound in the biological fluids of the patients and to calculate the pharmacokinetic parameters, is an essential tool for the optimization and future development of the drug in the clinical trial [5].
Pharmacokinetic studies with CIGB-300, in animal and human models, are based on the estimation of peptide concentration in the plasma through a radioactive method with a variant of the CIGB-300 conjugated to technetium 99 (99Tc) [3, 6]. Radioactive methods have, however, important limitations since they make it impossible to know how much of the radioactive signal recorded corresponds to the entire peptide and not its degradation residues. This could lead to overestimations of the true amount of the circulating molecule, thereby giving an incorrect evaluation of its pharmacokinetic parameters [7].
The ELISA system is one of the most widely used methods in clinical and analytical laboratories for pharmacokinetic studies [8]. It is very sensitive to the quantification of analytes in complex samples and it is not dangerous for human health [9]. Our group carried out a competitive ELISA assay using a polyclonal antibody generated against the CIGB-300, to capture this analyte found in the plasma. For its implementation, we first determined the parameters concerning its accuracy, precision, selectivity, sensitivity, reproducibility and the stability recommended for this type of analysis [10].
MATERIALS AND METHODS
Reagents
The Tween 20 reagent was purchased at Sigma Aldrich Labochemikalien GmbH, United Kingdom. Polyclonal antibodies anti-CIGB-300 developed in rabbits (Atlanbio, France) and anti-rabbit peroxidase (Sigma, Missouri, USA) were used. The substrate and stopping solutions for the immunoenzymatic reaction were purchased from R & D Systems (Minneapolis, USA), and the blocking solution Seablock at EastCoastBio (Maine, USA). The washing solution (0.05 % Tween 20) and the dilution solution (0.1 % Tween 20) and the 2 % fetal bovine serum (FBS; PAA, Canada), were prepared in a phosphate buffer saline (PBS; NaCl 1.37 M, KCl 27 mM, Na2HPO4 100 mM, KH2PO4 18 mM, pH 7.4) solution at 1×.
The peptide lot CIGB-300 P-300-02-0807 (CIGB, Havana, Cuba) was used as the reference material of the analyte.
Samples of human plasma
The samples of plasma from healthy donors were supplied by the Blood Bank of Vedado, Havana, Cuba. Those of cancer patients (vulvar carcinoma with lung metastasis, non-small cell lung adenocarcinoma, retroperitoneal cancer, rectum adenocarcinoma with muscle infiltration) were provided by the Center for Medical Surgical Research (Cimeq). The samples were stored at - 80 °C until thawing only once.
Immunoenzymatic assay
An ELISA/RIA plate (high binding polystyrene plates EIA, Costar, USA) was coated with 100 μL of CIGB-300 (reference material) dissolved at 1 μg/mL in phosphate buffer saline (PBS) 1×. It was incubated at 4 °C for 16 h and was later blocked with 200 μL of the Seablock blocking solution at room temperature (RT; 25 °C) in the dark for 2 h. Then, 50 μL of the serial dilutions of CIGB-300 (standard curve) were added in a concentration range of 0 to 10 μg/mL of the problem sample together with 50 μL of the polyclonal rabbit serum anti-CIGB-300 diluted at 1:10 000 in PBS 1×. The plate was incubated at RT in the dark for 2 h. Later, four washings were made with the solution PBS 1×-0.05 % Tween 20 and 100 μL of the anti-rabbit peroxidase conjugate diluted in the dilution buffer were added, and plates were further incubated at RT in the dark for 2 h. Afterwards, four washings were made with the PBS 1×-0.05 % Tween 20 solution, and 100 μL of the substrate solution were added per well and incubated for 30 min at RT in the dark. To stop the reaction, 100 μL of the stopping solution were added and the reading of the absorbancy was made at 450 nm (Abs450nm) in a SUMA plate reader, model PR-521 (Immunoassay Center, Cuba).
Finally, the value of the basic Abs450nm (sample without the CIGB-300 coating) was subtracted from the values of the Abs450nm of the assay for the calculations. The calibration curve was graphed with the quotient of the binding/maximum binding (B/Bmax) in the ordinates against the logarithm of the concentration of CIGB-300 in the abscissa, and the resulting equation was adjusted using a variable slope non-linear sigmoid model (Prisma program, version 4.0). The concentration of the CIGB-300 was obtained through the extrapolation of the individual value of the B/Bmax of each problem sample in the calibration curve. The Bmax was defined as the value that is equivalent to concentration zero of the CIGB-300 in the evaluated plasma; it is therefore the highest Abs450nm detected.
Quality parameters of the assay
Repeatability was verified through four curves of the same sample under identical conditions in the same assay and on the same day. For intermediate precision, four curves of the same samples under identical conditions were made in different assays and on different days.
Four calibration curves were calculated during validation in different days to determine the linearity and the working range (WR) of the system. Analyte concentrations in the experimental points of the curve were: 10, 2.5, 0.6, 0.15 and 0.039 μg/mL, respectively. The lower detection limit (LDL) was calculated as the Abs450nm (Bmax) minus two standard deviations (SD) of the system calibration curve (LDL = Bmax – 2SD). The upper detection limit (UDL) was calculated by interpolating the baseline Abs of the system (UDL = mean + SD). WR was established as the range of concentrations within the UDL and LDL of the system (LDL ≤ RT ≤ UDL).
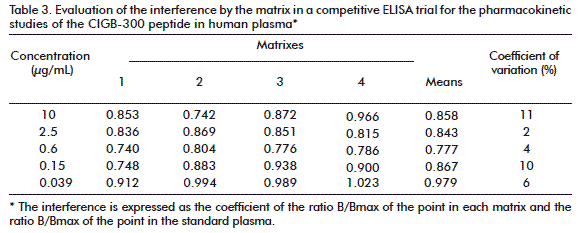
Specificity was determined as the capacity of the ELISA method to detect the analyte in a specific manner, in the presence of components that may be contained in the matrix and can interfere with the system. For this purpose, the plasma of four individuals was processed and known concentrations of the analyte were analyzed (10 μg/mL, 2.5 μg/mL, 0.6 μg/mL, 0.15 μg/mL, 0.039 μg/mL). The values of Abs450nm corresponding to each one of these samples were compared with the values of Abs450nm for this same concentration of the analyte in the standard curve.
To measure specificity, if the estimation of the concentration of the analyte is accurate, there will be no interference in the factor matrix system (FM), with a value of 1. The FM was calculated as the quotient of the response in the presence of the matrix analyte divided by the response in its absence. The variation coefficient (CV %) between them must be less or equal to 20 %, and therefore FM must be higher or equal to 0.8, and lower or equal to 1.2, for each one of the plasma samples analyzed in relation to the mixture, where the determination was made in the plasma of healthy donors (standard plasma).
The recovery of the system (REC) was determined using three concentrations of the analyte in the plasma of patients (n = 4) and in the standard plasma. It was calculated as the quotient between the Abs of different concentrations of the analyte (X) in the matrix that was to be evaluated, and the Abs of equivalent concentrations of the analyte in the standard plasma, according to the following formula:

For the analysis of analyte stability in the plasma, analyses of variance were carried out on the sample stored at - 80 °C, in the samples submitted to successive freeze-thaw cycles (up to 3 cycles at - 80 °C to RT), and of the analyte in the matrix without processing.
Stability of the sample stored at - 80 °C
The samples of the analyte in the plasma were prepared at three concentrations: high (10 μg/mL), medium (0.6 μg/mL) and low (0.039 μg/mL). Two replicates of each level of concentration at time 0 were analyzed in the samples prepared. The other samples were stored at - 80 °C and analyzed on days 18 and 30. The concentration of the initial determination was compared with the concentration values of the samples at medium-term and long-term storage.
Stability of the sample in the freeze-thaw cycles
The samples prepared at three concentrations were submitted to one, two or three freeze-thaw cycles (- 80 °C-RT). After each cycle, the sample was processed and analyzed according to the above description. The determinations were compared with the analyses of samples that were not submitted to this treatment and the experiment was duplicated for each concentration level in each experimental group. The results of the determinations were processed in the Prisma program (version 4.0).
Statistical analysis
The standard deviation and the coefficient of variation (%) were calculated using Excel 2007 from Microsoft® (Microsoft Corp.; USA).
RESULTS AND DISCUSSION
The CIGB-300 peptide is a new anti-tumor drug with a potent anti-neoplastic effect on pre-clinical cancer models [1], with a clinical evaluation currently in progress. This molecule inhibits phosphorylation through CK2, an oncologically validated molecular target [11]. In 2006 it was first evaluated in human beings, where it was directly administered in pre-malignant lesions of the cervix, and it showed to be safe and well tolerated [4]. The new oncological niches in which this molecule is explored are lung malignant neoplasia and acute myeloid leukemia (AML), in which the administration route must be systemic. The implementation of an analytical system that makes it possible to estimate the concentrations and calculate pharmacokinetic parameters in biological fluids is a main objective to optimize and develop this drug [5].
A radioactive method was used in the initial studies of the bioavailability of the CIGB-300, in which the peptide was conjugated to 99Tc [3, 6]. Although the concentrations of CIGB-300 reaching human plasma were calculated, as well as the preliminary correlations with the biodistribution variables, toxicity and signs of clinical efficacy, the disadvantages of this method are widely known [12, 13].
Immunoenzymatic assays have been developed as an alternative having the features of specificity, sensitivity, reproducibility and speed, among other advantages [14, 15], and making it possible to quantify peptides and proteins in biological matrices in a routine manner. This paper describes the process of the development and implementation of a competitive ELISA system for the determination of the CIGB-300 peptide in human plasma. It is the first method that makes it possible to monitor the presence of the complete molecule in biological fluids, in a relatively easy and reliable manner, and avoids the risks involved in the work with radioactive isotopes [16].
The ELISA system that was implemented made it possible to detect the analyte in the human plasma at the nanomolecular range, with an estimated LDL of 0.030 μg/mL. The determination of the detection limits is an important step for any analytical system, since it defines the minimum amount of analyte that may be quantified in a reliable manner in a biological fluid, and at the same time it determines the lower limit of the WR of the system [17]. A low detection limit makes it possible to track the peptide in the plasma for a longer period of time. A larger number of experimental points lead to a better estimation of the pharmacokinetic parameter of the drug under study. On the other hand, the estimation of the UDL from the dose-response curves obtained, indicated that the ELISA may detect concentrations of CIGB-300 in human plasma of up to 15 μg/mL, without the need of dilution. These determinations allowed for the establishment of a wide and reliable WR (from 0.039 to 10.0 μg/mL) for future evaluations, in order to estimate the concentrations of the analyte in plasma samples of cancer patients.
Other important parameters in establishing an analytical method are precision and accuracy; these are characteristics of yield that describe the size of random and systematic errors [18]. During the study of the intra-assay variability, the CV obtained was less than 5 % for each point of the curve (Table 1), while the values for intermediate precision were in the range of 14 to 17 % (Table 2). This means that the assay is reproducible and offers acceptable precision, according to the norm for this type of determination where the CV should be less than 20 % [19].
A ligand binding assay was used for the analysis of linearity, which uses the four-parameter logistic model (4PL). This type of test typically follows a non-linear sigmoidal association between the analyte concentration and the response. The model was chosen because it optimizes the precision and accuracy of the calibration range that is being used (of 5 to 8 concentrations that will be evaluated) [20, 21]. Calibration curves have a determination coefficient that is higher or equal to 0.98 (R2 ≥ 0.98) (Figure 1). The closer the R2 is to 1, the better the adjustment of the data to the model used, and this is an additional element in favor of the precision and accuracy of the test.
In order to establish the selectivity of the system, we evaluated the influence of the biological matrix used in the determinations. The values of the FM obtained in the plasma sample of the cancer patients, at each point in the curve, are in the range of 0.8 to 1.0 (Table 3). Although this result must be corroborated in a larger number of samples, it was demonstrated that the interferences that may be found in the plasma, such as proteins, lipids or the hemolysis of any of the samples, do not significantly affect the determination of the CIGB-300. Another measurement of the capacity of the system to detect the analyte is by studying the recovery. This is expressed as the ratio between the response obtained for the analyte in a sample (for example, the plasma of a patient) with the response obtained for that same amount of analyte in a standard matrix (for example the plasma of a healthy individual) expressed as percentage [18]. Three concentrations were analyzed: high (10 μg/mL), medium (0.6 μg/mL) and low (0.039 μg/mL), and the values obtained were 110.2, 98.9 and 119.8 %, respectively. The interference present in the sample has a positive influence on the recovery. For the extreme values of the curve, the analysis of the recovery indicates that the concentrations of CIGB-300 can be overestimated. However, these results must be interpreted with caution, since the values of recovery are located within the variability of the system.
Finally the stability of the sample was studied in two and three freeze-thaw cycles (- 80 °C-RT). Stability experiments must simulate the conditions in which the samples of the study will be collected, processed and stored. The stability of the sample may be assessed during the implementation phase of the method, for which studies at RT, from 2 to 8 °C, and the freeze-thaw cycle are generally included. These evaluations make it possible to define when the samples will be discarded from the life cycle of the system [17]. Several physicochemical analyses showed that the CIGB-300 molecule is not stable at 4 °C. For this reason, and considering the high number of proteases in the blood [22], our stability studies were carried out at - 80 °C. In the time intervals analyzed in relation to time zero (t0), the CV remained within the range of 3 to 6 % (Figure 2A), indicating that the same aliquot of the sample may be reevaluated at least three times without affecting the estimation of the analyte. In relation to the long-term stability, in the study of the sample stored for 18 days, the CV in relation to t0, remained in the range of 3 to 14 % (Figure 2B). For the sample stored for 30 days, the CV in the periods analyzed in relation to t0 remained at the range of 6 to 19 % (Figure 2B). In general, these results express that the analyte is stable in the human plasma during the time periods evaluated under these storage conditions. This finding is important from the operational viewpoint in the laboratory, since it offers a certain amount of flexibility to sample evaluation.
The quantification of the analytes through the ELISA type platform is generally simple, when dealing with an antibody having high specificity, as that of the antibody used in this study [5]. Several immunoenzymatic assays have been described for the detection of pharmaceuticals of peptide origin. One of them is a competitive ELISA, which is commercially available, and developed for the detection and quantification of the exenatide, a synthetic version of exendin-4. Exenatide is an antagonist of the glucagon-like peptide 1 (GLP-1 agonist) used to control glycemia, approved since 2005 [23] for the treatment of type II diabetes mellitus [24]. This system detects up to 0.1 μg/mL with an LDL of 0.00008 μg/mL [25]. Another competitive ELISA was designed to detect the presence of the hepcidin peptide, which contributes to the pathogenesis of chronic anemia. The system showed an LDL of 5.5 μg/mL, and a intra-assay variation of 8 to 15 %, and the intra-assay variation was of 5 to 16 %, with a recovery of 107 % [26]. Interestingly, the estimation of the LDL of our system showed that the assay is superior in this parameter compared to one of the ELISAs described (hepcidin), while the inter- and intra-assay variation had a similar behavior (CV < 20 %). Additionally, as a measure of selectivity the recoveries were compared and their values were found to be within the variation of 20 %.
Although the validation process for the ligand binding assay requires the introduction of other variables (for example: equipment, analysts, number of replicates, biological samples, among others) and data analysis methods [17], the implemented system is precise and accurate. The assay enables the quantification of the CIGB-300 peptide in human plasma, in a reliable and reproducible manner, without the possible estimation errors derived from the degradation of the peptide, which are frequent in the radioactive methods. At the same time, this stage of implementation made it possible to establish the critical reagents, the type of developer used in the system and the experimental design (i.e., the number of plates, the placement of the standards/validation samples, the number of repetitions, operation conditions and the size of the evaluation lots), which must be confirmed during the future validation stage [17].
Considering the advantages of an analytical type ELISA system described, the new method constitutes a robust, flexible and readily implemented alternative, to study the pharmacokinetics of the CIGB-300 peptide in future clinical trials.
ACKNOWLEDGEMENTS
We would like to thank Dr. Marta Ayala, Eng. Indira Pla, Cristina Rodríguez, MSc., Eng. Osvaldo Ávila Echemendía and the Validation Commission, all of which are from the Center of Genetic Engineering and Biotechnology of Havana, Cuba, for their contribution to this work.
REFERENCES
1. Perea SE, Reyes O, Puchades Y, Mendoza O, Vispo NS, Torrens I, et al. Antitumor effect of a novel proapoptotic peptide that impairs the phosphorylation by the protein kinase 2 (casein kinase 2). Cancer Res. 2004;64(19):7127-9.
2. Perera Y, Costales HC, Diaz Y, Reyes O, Farina HG, Mendez L, et al. Sensitivity of tumor cells towards CIGB-300 anticancer peptide relies on its nucleolar localization. J Pept Sci. 2012;18(4):215-23.
3. Perera Y, Farina HG, Hernandez I, Mendoza O, Serrano JM, Reyes O, et al. Systemic administration of a peptide that impairs the protein kinase (CK2) phosphorylation reduces solid tumor growth in mice. Int J Cancer. 2008;122(1):57-62.
4. Solares AM, Santana A, Baladron I, Valenzuela C, Gonzalez CA, Diaz A, et al. Safety and preliminary efficacy data of a novel casein kinase 2 (CK2) peptide inhibitor administered intralesionally at four dose levels in patients with cervical malignancies. BMC Cancer. 2009;9:146.
5. European Medicines Agency. Guideline on the clinical investigation of the pharmacokinetics of therapeutic proteins Committee for medicinal products for human use (CHMP). London: EMEA; 2007 [cited 2013 Nov 17]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003029.pdf.
6. Soriano-García JL, López-Díaz A, Solares-Asteasuainzarra M, Baladrón-Castrillo I, Batista-Albuerne N, García-García I, et al. Pharmacological and safety evaluation of CIGB-300, a casein kinase 2 inhibitor peptide, administered intralesionally to patients with cervical cancer stage IB2/II. J Cancer Res Ther. 2013;1(6):163-73.
7. Richter M, Cyranka U, Nowak G, Walsmann P. Pharmacokinetics of 125I-hirudin in rats and dogs. Folia Haematol Int Mag Klin Morphol Blutforsch. 1988;115(1-2):64-9.
8. Lequin RM. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem. 2005;51(12):2415-8.
9. Wild D, editor. The immunoassay Hand-book. 2nd ed. London: Nature Publishing Group; 2001.
10. Bansal S, DeStefano A. Key elements of bioanalytical method validation for small molecules. AAPS J. 2007;9(1):E109-14.
11. Seeber S, Issinger OG, Holm T, Kristensen LP, Guerra B. Validation of protein kinase CK2 as oncological target. Apoptosis. 2005;10(4):875-85.
12. Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960;39:1157-75.
13. Ekins RP. The estimation of thyroxine in human plasma by an electrophoretic technique. Clin Chim Acta. 1960;5:453-9.
14. Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8(9):871-4.
15. Van Weemen BK, Schuurs AH. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971;15(3):232-6.
16. Zakowicz, H. A 40-Year-Old Paradox: The Fascinating History of the ELISA. Boster Inmunoleader; 2013.
17. DeSilva B, Smith W, Weiner R, Kelley M, Smolec J, Lee B, et al. Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm Res. 2003;20(11):1885-900.
18. U.S. Department of Health and Human Services. Guidance for Industry: Bioanalytical Method Validation. Rockville: US Department of Health and Human Services; 2001 [cited 2013 Nov 17]. Available from: http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf
19. Kelley M, DeSilva B. Key elements of bioanalytical method validation for macromolecules. The AAPS J. 2007;9(2):E156-63.
20. Findlay JW, Dillard RF. Appropriate calibration curve fitting in ligand binding assays. The AAPS journal. 2007;9(2):E260-7.
21. Ministry of Health, Labour and Welfare of Japan. Draft Guideline on Bioanalytical Method (Ligand Binding Assay) Validation in Pharmaceutical Development. Tokyo: MHLW; 2014 [cited 2014 Jan 27]. Available from: http://www.nihs.go.jp/drug/BMV/BMV-LBA_draft_140124_E_rev.pdf
22. Matsui T, Imamura M, Oka H, Osajima K, Kimoto K, Kawasaki T, et al. Tissue distribution of antihypertensive dipeptide, Val-Tyr, after its single oral administration to spontaneously hypertensive rats. J Pept Sci. 2004;10(9):535-45.
23. Lax R, Meenan Ch. Challenges for Therapeutic Peptides Part 1: On the Inside, Looking Out. Innovations in Pharmaceutical Technology. 2012;(42):54-6.
24. Fineman M, Flanagan S, Taylor K, Aisporna M, Shen LZ, Mace KF, et al. Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing. Clin Pharmacokinet. 2011;50(1):65-74.
25. Creative Diagnostics. Heloderma Suspectum Exendin-4 ELISA Kit Cat. No.DEIA10583. New York: Creative Diagnostics; 2013 [cited 2013 Nov 17]. Available from: http://img2.creative-diagnostics.com/pdf/DEIA10583,Exendin-4.pdf
26. Koliaraki V, Marinou M, Vassilakopoulos TP, Vavourakis E, Tsochatzis E, Pangalis GA, et al. A novel immunological assay for hepcidin quantification in human serum. PLoS One. 2009;4(2):e4581.
Received in January, 2014.
Accepted in July, 2014.
Vilcy Reyes. Laboratorio de Oncología Molecular, Subdirección de Investigaciones Biomédicas, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba. E-mail: vilcy.reyes@cigb.edu.cu.