My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.31 no.4 La Habana Oct.-Dec. 2014
FOCUS
On the negotiation of biotechnology products that include intangible assets
Sobre la negociación de los productos biotecnológicos con intangibles asociados
Dora García Delgado1, Maritza Ortiz Torres2
1 Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 entre 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
2 Facultad de Economía de la Universidad de la Habana. Calle L entre 23 y 21 Vedado, La Habana, Cuba.
ABSTRACT
In biotechnology, products negotiations are imposed by the intangible component associated to them, which can be either due to the strength of the patent, its novel technology or the fact that these concern research and development projects, at the different stages of development of a biotech product. This paper covers some general topics on negotiations that are included in any process: negotiation stages, strategies, principles and settings. An explanation is also given on the negotiation of intangible assets: patents, trademarks, technologies and commercial rights, the meaning of upfront fees and royalties on sales. The results of the analysis of a sample of the negotiations in BioCubaFarma (the Cuban biotechnology and pharmaceutical industries group) with intangible assets are given and compared to another study of a sample representing more than 1500 negotiations of biotechnology companies occurring between 1998 and 2003. The conclusions of the comparison show that the upfront fees in this sector are in the same range as the international negotiations of the study taken as a reference. In the case of royalties, the average percentage is very close to the international mean. The analyses show that within the negotiations associated with intangible assets in BioCubaFarma there is a prevalence of licensing agreements for co-development and subsequent marketing, followed by distribution agreements. Finally, the most frequently used business models in the Cuban biotechnology industry are described.
Keywords: pre-commercial payments, upfront payments, milestones, royalties.
RESUMEN
La negociación de los productos biotecnológicos es impuesta por el componente intangible asociado, ya sea por fortaleza de patente, por tener tecnología novedosa o por tratarse de proyectos de investigación y desarrollo, en las diversas etapas del desarrollo de un producto. En este artículo se describen los elementos comunes a cualquier proceso negociador: las etapas de la negociación, las estrategias, los principios y escenarios. Además se ofrece una explicación acerca de la negociación de los activos intangibles: patente, marca, tecnología y derechos comerciales, qué son los pagos precomerciales y las regalías sobre las ventas. Se exponen los resultados del análisis de una muestra de negociaciones con activos intangibles en BioCubaFarma (grupo cubano de las industrias biotecnológicas y farmacéuticas), y se compara con otro estudio de una muestra representativa de más de 1500 negocios de compañías biotecnológicas, sucedidos entre 1998 y 2003. De la comparación se concluye que los pagos precomerciales en el sector se mueven en el mismo rango de las negociaciones internacionales del estudio tomado como referencia. En el caso de las regalías, la media de su porcentaje es muy cercana a la media internacional. El propio análisis arroja que entre los negocios con activos intangibles asociados en BioCubaFarma predominan los acuerdos de licencia para codesarrollo y posterior comercialización, seguidos por los acuerdos de distribución. Finalmente se describen los modelos de negocios más usados en la industria biotecnológica cubana.
Palabras clave: pagos precomerciales, pago anticipado, hitos, regalías.
INTRODUCTION
Negotiations began ever since human beings felt the need to communicate with other human beings, and they saw themselves in the obligation of giving in products, values or goods to obtain something in exchange. Hence, the negotiation process is almost as old as humanity itself.
In the negotiation of goods, the products transfer their specificities to the negotiating processes. For example, there are products that are quoted on the stock exchange and there are elements in them that are specific to the negotiations of stock exchange goods. In the negotiation of the biotechnological products, the amount is given by their intangible value.
The aim of this paper is to offer information on the total amount of upfront payments and royalties on sales in the biotechnology industry, since these are characteristic elements of the negotiations of products within this sector.
The payments may potentially generate incomes that are at times not received because the intangible assets are not negotiated. For example, if the commercial rights of a novel biotechnology product with a patent are given in a country where that patent has been applied for or conceded, and nothing is requested in exchange, incomes are not received. If biotechnology research projects are negotiated without knowing the stage of the negotiation at which the highest amounts of money are requested: whether in the pre-marketing stage or as a royalty on the value of the sales, money can also be lost. This shows the importance of correctly assessing the intangible assets and preparing the negotiation appropriately.
AN OVERALL VIEW OF THE NEGOTIATION
In a negotiation, the parties involve solve conflicts, search for individual or collective advantages, trying to obtain results that cover their interests, which usually lead to a possible alternative for conflict solution, both in the business world as in life. But, what is a negotiation?
Roger Fisher and William Ury were more categorical when considering that whether you like it or not, you are a negotiator. The negotiation is a basic means of achieving what we want from others. It is a communication of an alternative route to reach an agreement, when you and another person share certain interests in common, but when there are also other opposed interests [1].
Several authors such as Nieremberg, Desaunay, Schatzki, Villalba, Deulofeu and Maritza Ortiz offer definitions of the term “negotiation”. These definitions have aspects in common, such as the existence of two or more parties with common and opposed in-terests, the need for communication and for reaching a final agreement [2].
The negotiation can also be defined as a process of information exchange between two or more parties to conciliate their interests, in order to reach an agreement and as a commercial negotiation. It is the mean through which a buyer and a seller, through a specific type of communication, i. e. commercial bargaining, reach an agreement on the type of contract, which must show a certain balance between the interests of both parties [3].
The negotiation should be considered a process covering several stages. In the consulted bibliography there are several classifications. Ortiz classifies them in five stages: 1) Preparation; 2) Interaction; 3) Bargaining and concessions; 4) Agreements and conclusions; and 5) Evaluation (Figure) [2].
It is important to point out that the negotiating process is based on the following principles:
1. To cover the needs more than the desires;
2. Fix ambitious but realistic goals;
3. Know the reach and strength of power of each partner and use this appropriately;
4. Listen to the partner;
5. State the negotiation proposal in an advantageous manner for both partners;
6. Manage the information with skill; and
7. Choose the appropriate strategy and be willing to change it when necessary [2].
For the success of the negotiating process, certain variables must be known and managed correctly. The principles are the relations of power of the parties, negotiation time and information [2].
The manner in which each party conducts the negotiation process must be known in order to reach the proposed objectives. This is called the negotiation strategy, and it must be defined at the preparation stage. Two typical strategies are known:
The “win-win” strategy that is integrative and collaborative: It tries to have both parties win, reaching a mutually profitable agreement and sharing the profits.
The “win-lose” or distributive strategy: Each party tries to attain the maximum profit without any concern on the situation of the opponent. In contrast to the previous strategy, the process is carried out in a confronting environment, in which the other party is seen as a rival that must be defeated.
It is important to point out that there is in fact no negotiation having a purely integrative or distributive strategy, but a combination of both, depending on each one of the aspects of the negotiation that is being analyzed.
During the preparation stage for the negotiation there should be several scenarios, since rigid objectives will only hamper their reach:
Optimistic scenario: It is the best result possible corresponding to that presented in the business offer or proposal.
Intermediate scenario: It is found to have below optimum results, which is good enough for the deal to be closed.
Pessimistic scenario: It is the point, up to which each partner is willing to accept, and below which no agreement is of interest; it should correspond with the approved business directives.
During stages 2 and 3 of a negotiation the parties normally reach a dead end, which does not mean that an agreement cannot be reached. In a negotiation this is known as blocking.
To overcome the blocking, new alternatives must be found using different approaches and different highly useful techniques. William Ury [4] has proposed the negotiation technique of doing the opposite of what the partner expects us to do. This means that if the partner gets rigid, they are expecting pressure; if their strategy is that of attacking, they expect resistance. What should be done is not to press or to resist, but to do exactly the opposite of what they expect us to do.
Another technique is placing yourself on the side of the partner, which is the last thing they expect you to do. This creates confusion and makes it possible for them to open and change their position. As Ury points out, it is difficult to attack someone who suddenly gets on your side [4]. This technique places both parties on the same side, enabling them to restart the negotiation in a constructive manner.
Something that must not be left out is that of making concessions: these are the driving forces of negotiations. The way and the time they are presented are of fundamental importance because the possibility or not of reaching an agreement may depend on this. Concessions are used in the search for an immediate objective at the time when they may have the greatest impact, and not in a random manner.
When making the concessions, the type of concessions that you are willing to make when you decide to make them, must be clear. On the other hand, it is fundamental to assess the cost of the concession and try to offer those that are more economically convenient, but that at the same time may be important for the opponent.
NEGOTIATING BIOTECHNOLOGICAL PRODUCTS
The negotiation of biotechnological products is essentially the same as any other negotiating process and it goes through the same stages, with the peculiarity that they are associated to intangible elements of great value, which must be taken into account. These intangible elements are patents, technologies and trademarks, which are normally licensed. Furthermore, in the biotechnology industry the projects are frequently negotiated during different stages of development of the product.
Part of the value of the intangible assets mentioned and of the research and development projects is received through payments before the product reaches its commercial phase; these are therefore called upfront fees. The other part of the value is received during the commercial phase as royalties on sales. Both elements are explained below.
Upfront fees
The risk a business executive is willing to run for a project demonstrates their interest in it; therefore the upfront fees are used to fund the pending stages of development of the product.
Steve Poile and Suzanne Elvidge [5] described the average sums that should be paid as upfront fees, according to goals or milestones, and the percentage of royalties. The sources they used to design these data were:
1. Royalty Source (www.royaltysource.com): a tool for professionals involved in intellectual property assessment. The data covers 61 negotiations between January 1998 and February 2 of 2003.
2. Recap Alliance (www.recap.com): a source of public bio-pharmaceutical negotiations. This covered 1420 negotiations performed between January 1998 and April 2003.
These authors defined upfront fees, considering them payments in advance and the payments for meeting milestones or goals. That is to say payments for rights acquired at the start of an agreement, generally paid when signing the contract.
This payment shows the trust of the license holder and the need of funding of the licensee. In the first stages of the projects, the license holder tries to always avoid it whenever possible, because of the risks involved. This is shown in the low amounts given by the mean and the median. Only 28 % of all business deals reviewed in this study paid upfront fees, and these were in the range of 10 000 to 2 million US dollars (USD), with a mean of 460 000 USD and a median of 100 000 USD [5].
The milestones or goals mark the stages defined within the course of a project: patent presentation, patent granting, the start of preclinical trials, the successful end of preclinical trials, and approval by the regulatory authorities in specific markets [5].
The amount of upfront fees and of the fees given when goals or milestones are met depends on the stage of development of a biotechnological product. For a project that has completed phase II and demonstrated the efficacy of the product, the payments can be five times higher than the same project after ending phase I [6].
In the case of technologies, these goals or milestones are associated to having completed the stages for the technology transfer.
In the case of products with a strong patent during the commercial state, these payments are also made, basically in the licensing of the patent for their commercial operations. In these cases, the milestones or goals are associated to the stages of the product allowing it to be sold in the market. These stages are: the presentation of the dossier for the sanitary registration before the regulatory authority of the country, approval by the regulatory authority, launching the product and starting the sales.
Kolchinsky describes the negotiation of the biotechnological products in two ways [6]:
1. Projects in early stages of development.
2. Projects in advanced stages of development.
In the case of projects in their early stages of development, in which the risk is high and funding is needed to conclude the development of the drug, the highest amounts are paid as upfront fees: payment in advance and payments when goals are met. The value left to pay will be paid as royalties on sales.
Because of the high risk, the payments during the initial stages of the project are much lower than those of the final stages. In exchange to sharing a higher risk, the partner will have a higher profit.
For example: there are small companies that try to license candidates at the preclinical phase where the risk is high; the partner may offer 100 000 USD as an upfront payment when signing the contract; 0.5 million USD at the start of the preclinical phases; 0.5 million at the start of the phase I clinical trial; 1 million at the start of the phase II clinical trial; and 3 million at the approval by the regulatory authority. It is observed that the payment during the first three stages sums up to 1.1 million USD, and at the last two stages this is 4 million USD, almost four times the value paid during the initial stages.
In the case of the projects that are in advanced stages of development, where the risk has decreased, the lowest sums are offered as upfront payments and the highest amount is paid as royalties. In the pre-marketing payments the highest amounts are paid during the initial stages.
For example: in a phase III study that has concluded with positive results, the pre-marketing payments are of small amounts and the payments due to royalties are high. Among the pre-marketing payments, the highest amounts of money are requested in the early stages. Only one high pre-marketing payment may be requested, which is normally an upfront fee.
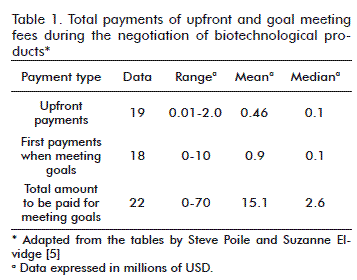
The first payment when meeting the goals may reach up to 10 million USD, although most of them reach a maximum of half a million, with a mean of 900 000 USD and a median of 100 000 USD. The total amount paid for meeting the goals may reach up to 70 million USD, with a mean of 15.1 million USD and a median of 2.6 million USD (Table 1) [5].
In relation to the amount of payments for when goals are met, this study shows that this can range from between 2 and 10 million USD, and therefore the mean and the median both equal 5 million USD [5].
Royalties
Royalties are payments made as percentages on sales [5], for which reason one of the most important factors affecting the total amount paid as royalty is the definition of the sales. The definition of whether the royalty is calculated on gross sales or net sales is essential.
Other authors define net sales as those calculated according to the price of the product, without considering the margins of the distributors [5]. Some authors define them as sales, after deducting the taxes. Therefore, the sales concept on which the royalties will be calculated must be defined during the commercial operation.
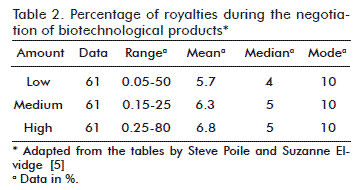
The percentages of royalties may be fixed or scaled, depending on the agreements in the contracts of specific markets, considering the local regulations [5]. Table 2 summarizes the percentage of royalties resulting from the study made by Poile and Elvidge.
As observed in table 2, most of the negotiations have rates of royalties of 10 % or less, with a mean of 5.7 %, for a range of 0.05-50 %; a mean of 6.3 % for a range of 0.15-25 %; and a mean of 6.8 % for a range of 0.25-80 %. When considering the frequency of the mean rate of the royalties for a higher precision, it is found that the rates of royalties range from 0 to 21.9 % with a peak at between 4.0 and 6.0 % [5].
Considering the representativeness of the sample, the source and the depth of the statistical analysis, which considered the mean, the median and the mode, the range proposed is from 0 to 20 % to determine the percentages of the royalties in the biotechnology products business.
Other analyses of Poile and Elvidge show that the rates of royalties of 50 % correspond to the profit sharing business models or to joint ventures.
A sample of thirteen negotiations in enterprises of BioCubaFarma was taken: five were from the CIMAB S. A. enterprise, six from Heber Biotec S. A., one from Dalmer Laboratories and one from Vacunas Finlay S.A. The parameters analyzed were: type of agreement, amount paid in upfront payments, number of goals, amount paid for meeting the goals in the first payment, amount paid for meeting the goals in the last payment and total amount of payments made for meeting the goals, as well as the percentages of royalties.
Within the 13 agreements there were 8 licensing agreements for co-development and later marketing, which represents 62 % of the sample; and 5 are distribution agreements, in which the commercial rights are licensed, representing 38 %. In 5 of these 13 agreements the licensing of the technology for local production is clearly expressed, representing 38 % of the sample.
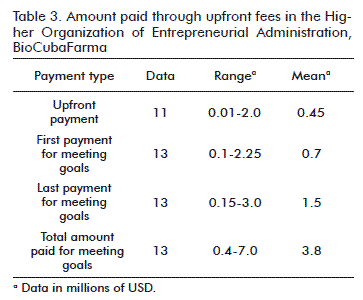
On the basis of the data collected, the total amounts of payments made are summarized (Table 3).
The upfront payments ranged from 10 000 to 2 million USD. Coincidently, the same result was obtained by Steve Poile and Suzanne Elvidge [5]. The amount we paid for meeting the goals is also in the same range as the amounts reported by these authors.
In relation to the number of payments fixed for meeting goals, the mean of Poile and Elvidge [5] is of 5 and the mean in the sample of BioCubaFarma is of 4 (Table 4).
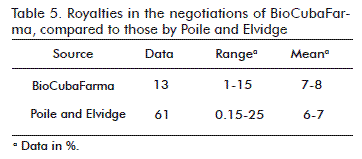
Table 5 shows the comparisons of percentages of royalties that are derived from the analysis of the negotiations in BioCubaFarma, with those presented by Poile and Elvidge [5]. The range of these authors was greater but the mean was very close to ours. The mean of the royalties in BioCubaFarma was of between 6 and 7 %, and that of the authors is within 7 and 8 %.
Now we will see the specificities of the negotiations of the assets: trademarks, technology and commercial rights, the latter derived from the commercial use of the patent.
Trademark
A sample of 10 cases of international negotiations in which the terms were published in Biopharmaceutiques [7] was analyzed, and it was observed that in no case did they license the trademark asset. In the sample of the negotiations of BioCubaFarma only in two cases was this asset licensed and in one of the two it was agreed to grant it free of charge.
This may be due to the fact that in the pharmaceutical and biotechnological industries the prestige of the manufacturers or the marketing companies is what differentiates the products, even more than the trademarks. For example, the consumer demands Bayer aspirin and Shering or Roche interferon or vaccines from GlaxoSmithKline, regardless of the commercial name of the products.
However, when it is a novel product with a registered trademark in the target market, this asset is considered and included in the negotiation proposal. Regardless of the strength of the trademark, of the strategy it is made to follow and of the specific ne-gotiation, the payment for the trademark will or will not be demanded. If before, or during the negotiation process it is decided that the trademark is not to be negotiated, this should remain as a contribution, or it is to be used as a concession, in case there is blocking during the negotiation.
Technology
The technology may be completely licensed or only some stages. The value should be calculated. The negotiation of a technology transfer is a complex negotiating process in which the timetable of the transfer is well designed, as well as the reach of the technological packages and technical assistances, and also the payments of the value of the technology, considering that the fixed goals for each stage of the transfer are met. On concluding the negotiation, the technology transfer is signed.
Commercial or operational rights of the patent: license for use
Partners frequently demand commercial rights for the distribution of the patented product in one or several countries where the patent has been applied for or granted. On granting this right they are yielding the right to operate this patent commercially.
To negotiate these rights the cash flows that are expected through the sales of the product for each market in which the commercial rights are granted must be calculated separately. A strategic analysis must be carried out in the places requested and several scenarios are prepared through which new places are yielded gradually as the performance of the partner is shown in those already granted. The upfront fees are requested when granting commercial rights; the goals are given by obtaining the sanitary registration and implementing the sales plans in each place.
NEGOTIATION MODELS FOR BIOTECHNOLOGICAL PRODUCTS
The Cuban biotechnology experience has been to implement a convenient combination of the complete development strategy of the products (to negotiate only the commercial representation), with early pre-marketing negotiation strategies (for the joint development of products) [8]. Starting with the negotiation strategy that is in general applied in Cuban biotechnology [8], the negotiation models with which this strategy is materialized, are described.
Agreements for licensing, representation and distribution of a novel final product
The intangible assets that should be taken into account in the negotiation of agreements for the distribution of patented novel products, with the commercial rights through the commercial operation of the patent (license to operate the patent and the trademark.
The right granted to sell a patented product in a place is equivalent to the licensing of that patent for its commercial operation, for which reason the license holder should evaluate that license and establish the payments for the commercial rights that they are licensing.
After calculating the value of commercial rights, the payments are established according to goals or milestones of the commercial regulating type, such as obtaining the sanitary registration in a certain market, commercially launching the product and starting the sales.
The patent license is a contract through which the licenser (maintaining their condition as the owner of the patent, by means of a remuneration, and for an established period of time) authorizes the license holder to exert all or some of the faculties attributed to them by legally being the owner of the patent, which includes the distribution of the product protected by a patent.
In an agreement for representation, licensing and distribution of a final product there is no risk in clinical development. A design can be applied in which the highest amount is paid as royalties on net sales; and among the upfront fees, the highest amounts can be paid at the start of the upfront fee payments. There is often only one payment in advance and the rest of the intangible value is paid as a royalty.
Because there are new products with patents, the prices are high according to the savings that the new therapy can represent for the patient. The price is not fixed according to the cost of production, but above it.
In the licensing agreements the technologies, patents and trademarks are licensed; a company completely transfers the risk of a project to another company in exchange for payments. While in the licensing agreements the rights are transferred from one partner to another; in the partnerships both parties are important in the development and marketing of the product and they share risks and profits.
The co-development agreements lead to partnerships where foreign partners are involved. In Cuba, the Cuban partner receives less than when the entire development is carried out without foreign participation, since one part of the value of the product is transferred to the partner, depending on their participation in product development. This is so when that participation is absolutely necessary to obtain something that is required at that time to complete the development, which can include productive facilities, production standards and regulations or funding to be able to end the process on time so that the product can be appropriately placed on the market.
In these contracts, the foreign partner contributes with venture capital for continuing and completing the project, and upfront payments are made, of which the amount considers the intangible value created by the Cuban partner. They receive in exchange the commercial rights in certain places, which will be effective if the project finally generates a marketable product [8].
In this co-development mode there are intangible assets for patents, commercial law and technology.
Technology transfer
Technology transfer is a business modality in which the partner wins in technology and the owner ensures a market during the technology transfer period. In the framework of this type of agreement the supply for the market can be guaranteed by the facilities of the partner, while increasing productive capacity and productive standards. This negotiation is generally carried out when the partner ensures large markets, such as the supplies for health ministries to meet the needs of the domestic health programs.
The intangible asset in this modality is the technology. Its value should be calculated by the technological factor method (tech factor), consisting in applying to the current net worth (CNW) adjusted to the deducted cash flow risk that is generated by that technology: an amount calculated according to the weighting of the attributes of that technology.
The payments are negotiated according to technological goals or milestones, determined by the stages of the transfer, as for example the delivery of technological packages, the production of test lots, etc.
Manufacturing contract
The manufacturing contract is a modality that corresponds to the need of a higher productive capacity or standard, making it necessary to search for productive solutions abroad. A manufacturing service is contracted, which involves licensing the technology [6].
Co-promotion and co-marketing
The terms co-promotion and co-marketing are often used as synonyms, but there are differences between these two modalities. In co-promotion, two companies deploy their sales forces under the same trademark, as a way of collaboration. In co-marketing, each one sells under a different trademark, as if these were two different products, thus avoiding the competition between them. This second modality is rarely applied because it is difficult to carry out. An example of this is the erythropoietin (EPO) from Amgen: they sell EPO (EPOGEN®) in the United States as a medication for kidney disorders (a segment of the market). Amgen licensed EPO to J & J, who sells it in the United States under the name Procrit® as a medication for cancer. In markets other than the United States, it is sold for all of its medical indications.
Sharing profits
In the process of developing a product, a company may share development and commercial costs, such as when launching the product, in exchange to sharing their participation proportionally in the profits (sharing profit). Both parties assume the risk that the product might never be approved, and in spite of sharing profits, the partner will pay in advance and after meeting their goals, which will make it possible to complete the development of the product and its marketing. The profits will be shared in the proportion agreed upon by the parties [6].
Joint ventures
Certain negotiations agree on the creation of a third entity called a joint venture. Each partner of this enterprise is the owner of one part of it. It is created by one or both partners, operated by scientific and technical personnel of one or both partners, and the joint venture receives a license from one or both partners to use a technology [6].
Due to the use of the personnel, the company pays one or both partners. Also, the company may license a technology to a partner and receive royalties for this.
A joint venture is an accounting facility where the partners can receive profits through direct transactions, depending on the influence of the company in their financial situation [6].
Binding agreements
In a negotiation, the goal of both parties is to sign an agreement that defines the future rights and obligations, and that specifies in detail what would happen if the agreement would be dissolved. Sometimes the buyer does not completely rely on the success of the biotechnological product they will acquire. For this reason, they may ask for a test sample under the condition that if this does not meet their expectation, the partnership is over. This takes place under a binding agreement. The condition is that the seller cannot engage another partner in the negotiation during the test period. The seller may claim for compensation because of opportunity costs.
CONCLUSIONS
On negotiating biotechnological products these go through the same stages as any other negotiation. The same principles and strategies are applicable; although in these negotiations the upfront payments and royalties on sales are specific. The value of the upfront payments in BioCubaFarma is in the same range as those of international negotiations. The mean of the percentage of royalties here is very close to the international mean. In BioCubaFarma the licensing agreements for co-development and later marketing prevail in the negotiations of associated intangible assets. The trademark is the least negotiated intangible asset.
REFERENCES
1. Fisher R, Ury W. Sí, de acuerdo. Cómo negociar sin ceder. Bogotá: Editorial Norma; 2005.
2. Ortiz M. Fundamentos de negociación. La Habana: Universidad de La Habana; 2010.
3. Le Bail C. La negociación de Compra. Barcelona: EUSA; 2000.
4. Ury W. El arte de negociar en situaciones difíciles. Chicago: Adventure Works Press; 1985.
5. Poile S, Elvidge S. Early stage and discovery deals: Strategy, structure and payment. London: Bridgehead Pharmalicensing Group Ltd.; 2003.
6. Kolchinsky P. The Entrepreneur’s Guide to a Biotech Startup. 2004 [cited 2014 Jul 12]. Available from: www.evelexa.com.
7. Biopharmaceutiques Merging Pharma & Biotech. 2009 [cited 2014 Jul 8]. Available from: http://www.biopharmaceutiques.com/en/tables/agreements/index.html.
8. Lage A. La Economía del Conocimiento y el Socialismo. La Habana: Editorial Academia; 2013.
Received in July, 2014.
Accepted in November, 2014.
Dora García Delgado. Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 entre 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba. E-mail: dora.garcia@heber-biotec.com.