My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.33 no.4 La Habana Oct.-Dec. 2016
RESEARCH
Micropropagation of garlic (Allium sativum L.) and determination of the genetic stability of the plantlets obtained by AFLP markers
Micropropagación del ajo (Allium sativum L.) y determinación de la estabilidad genética de las plántulas obtenidas mediante marcadores AFLP
Humberto Izquierdo-Oviedo1, Rosalina Disotuar2, María C González1, Sergio J González3
1 Instituto Nacional de Ciencias Agrícolas, INCA. Carretera a Tapaste, Km. 3 ½, San José de las Lajas, CP 32700, Mayabeque, Cuba.
2 Instituto Finlay. Ave. 27 No. 19805, La Coronela, C.P. 16017, La Lisa, La Habana, Cuba.
3 Facultad de Biología, Universidad de La Habana. Calle 25 e/n J e I, Vedado, CP 10400, Plaza de la Revolución, La Habana, Cuba.
ABSTRACT
This work was aimed to establish a methodology for the micropropagation of garlic (Allium sativum L.) ‘Criollo-9’ clone, to assess different combinations of AFLP primers to determine the genetic stability of vitroplants and to assess morphoagronomically the regenerants that were obtained in vitro. Cloves showing no signs of disease and under asepsis conditions were selected; the caudal apices were extracted between 5-10 mm and growth and development were assessed, both in vitro and ex vitro. As results, with the MS medium with NAA (0.1 mg/L) and Kinetin (0.1 mg/L) the cauline apices reached a higher percentage of establishment, in a shorter time; the highest number of shoots (20) was emitted in a medium enriched with NAA (0.1 mg/L) and 2-iP (4 mg/L) and for the first time in Cuba, AFLP markers were used in garlic and with the different combinations of primers that were used variability was not detected in the regenerants obtained. The percentage of in vitro bulbification was higher and was achieved in a shorter time in MS medium without growth regulators. The acclimatization of the microbulbils was higher than 99 % in a substrate containing zeolite (25 %) and organic matter (75 %). Under field conditions, yields higher than 12 t/ha were obtained and the results with molecular markers were corroborated, so the micropropagation technique is feasible to apply to this garlic genotype.
Keywords: In vitro garlic propagation, molecular markers, crop yield, substrates.
RESUMEN
El objetivo de este trabajo fue establecer una metodología para la micropropagación del ajo (Allium sativum L.) clon ‘Criollo-9’, evaluar diferentes combinaciones de iniciadores AFLP para determinar la estabilidad genética de las vitroplantas, y los regenerantes que se obtuvieron in vitro según caracteres morfoagronómicos. Se seleccionaron dientes de ajo que no presentaban signos de enfermedad. En condiciones de asepsia se extrajeron los ápices caulinares entre 5-10 mm y se evaluó su crecimiento y desarrollo, tanto in vitro como ex vitro. Los ápices caulinares crecieron más rápido y mostraron un mayor porcentaje de establecimiento con el medio MS con ANA (0.1 mg/L) y Kinetina (0.1 mg/L); el mayor número de brotes (20) lo emitieron en un medio enriquecido con ANA (0.1 mg/L) y 2-iP (4 mg/L). Por primera vez en Cuba, se emplearon los marcadores AFLP en ajo, y con las diferentes combinaciones de cebadores que se emplearon no se detectó variabilidad en los regenerantes obtenidos. El porcentaje de bulbificación in vitro fue superior y se logró en un menor tiempo en un medio MS sin reguladores del crecimiento. La aclimatización de los microbulbillos fue superior al 99 % en un sustrato que contenía Zeolita (25 %) y materia orgánica (75 %). Se obtuvieron rendimientos superiores a las 12 t/ha en condiciones de campo y se corroboraron los resultados obtenidos con los marcadores moleculares. Por tales razones, se demuestra que la técnica de micropropagación es factible para la propagación y cultivo del ajo de este genotipo con altos rendimientos.
Palabras clave: propagación in vitro, marcadores moleculares, rendimiento de cultivo, sustratos.
INTRODUCTION
Garlic (Allium sativum L.) is among the earliest plants consumed by man for culinary or medicinal purposes [1]. Some beneficial compounds for health, such as essential oils, anthocyanins, prostaglandins, fructans, pectins, oligosaccharides, adenosine and vitamins, among others, are found in leaves and bulbs, but allicin is the main responsible for its medicinal properties [2]. Regular consumption of this vegetable prevents diabetes, asthma, cardiovascular diseases and cancer [3].
Production in the world increased by 36 %, with an annual average above 17 million tons and an average yield of 11.78 t/ha [4]. This species has great economic importance for our country, since its demand by the population grows more every day; however, numerous efforts are being made to obtain new genotypes better adapted to our environmental conditions and to ensure yields above 6 t/ha, due to the difficulties encountered with its cultivation [5].
Genetic improvement by traditional methods is very difficult, since it is a species of asexual reproduction and strict apomixis, reason why the only route of propagation is by the microbulbils or cloves that are formed annually in the bulb [6]; every year cloves are sown from bulbs harvested the previous year in order to obtain new harvests. Under these conditions, phytopathogenic diseases are more easily transmitted to the offspring, which causes a progressive and irreversible weakening of clones or cultivars. There are several phytosanitary problems capable of causing losses of 50 % or more in yields, either due to the presence of diseases caused by nematodes, fungi, bacteria or viruses, these last the most influential in the deterioration of the crop. The onion yellow dwarf polyvirus (OYDV), the leek yellow striatum virus (LYSV) and the shallot latte virus (SLV) are the most frequent, and the first of them the most difficult to eradicate [7, 8]. This is where the use of biotechnological techniques could have a substantial impact.
The development and application of biotechnology has great importance for garlic, which allows micropropagation of clones previously cleaned from viruses and obtaining basic seed of high biological quality.
Biotechnological methods are applied to garlic in different countries, aiming at obtaining and characterizing improved genotypes and providing a vegetative propagation technique that facilitates the maintenance of materials of interest [1, 7]. Nonetheless, these results are not always extrapolated, which may be due to differences in the genetic and physiological characteristics of the clones or cultivars grown and their adaptation to other ecological conditions.
Morphological [9], physiological [10], cytogenetic [11], and isoenzyme markers [12] are used to determine variations in the genome of Allium sativum L. plants obtained in vitro. Yet, these descriptors may have some disadvantages such as being subjected to environmental changes, which at a given time can modify the expression of a genotype, do not reveal alterations of chromosomes [11] at specific sites, or have a low level of polymorphism and may not show the genetic changes occurring in DNA [13].
Taking into account advances in biotechnology, techniques in which molecular markers are used allow identifying variations or polymorphisms by differences in DNA sequence [14]. These markers are not affected by the environment [13, 14]. Among them, the Amplified Fragment Length Polymorphism (AFLP) technique accurately detects the variation in many species of the Alliaceae family [12]. AFLPs are very powerful because they can be used without prior characterization of the genome sequence. Moreover, these markers detect a high number of loci not identified by other markers based on the random-amplified polymorphic DNA (RAPD) technique [12, 13].
Hence, this work was aimed to establish a methodology for the micropropagation of the garlic ‘Criollo-9’ clone, based on the evaluation of different combinations of AFLP primers to determine the genetic stability of vitroplants and to assess morphoagronomically the plants obtained by in vitro culture.
MATERIALS AND METHODS
General procedure for the study of the different phases for micropropagation
Vegetal material
The start planting material was obtained from garlic (Allium sativum L.) bulbs of the ‘Criollo-9’ clone, which had no apparent signs of phytopathogenic disease and had previously been cleared from viruses. In order to start the in vitro culture, cauline apices between 5-10 mm-long were used as the source of explants. All laboratory work was performed under sterile conditions.
Culture media
The Murashige and Skoog (MS) medium [15] was used as basal medium and supplemented with thiamine HCl (0.4 mg/L), nicotinic acid (0.5 mg/L), pyridoxine HCl (0.5 mg/L), glycine (2 mg/L), adenine sulfate (80 mg/L), myoinositol (100 mg/L), agar (6.5 g/L) and sucrose (30 g/L). pH was adjusted to 5.7 before sterilization by autoclaving at 121 °C and 1.5 atm for 15 min.
Culture conditions
Cultures were incubated at a temperature of 22 ± 2 °C, 35 μmol/m2•s and a photoperiod of 16 light hours.
Acclimatization of material from in vitro culture
The acclimatization was performed in a greenhouse, in plastic trays of 72 holes and a capacity of 47.61 cm3. Substrates were prepared with charged zeolite (Litonite) and decomposed filter cake (organic matter). All technical requirements were followed according to Izquierdo et al. [16].
Statistical analysis
The experiments were repeated three times and all data were used for each of the indicators assessed. A completely randomized design was used and data were processed by one-way ANOVA and the means were compared according to Tukey’s test (p ≤ 0.05). The statistical package STATGRAPHICS Plus version 5.0 for Windows was used for data processing. In all cases, the normal distribution (Kolmogorov-Smirnov test) and homogeneity of variance (Bartlett’s test) were previously checked.
Study of different phases for micropropagation
Influence of growth regulators on the in vitro establishment of explants
Explants were inoculated into a culture medium enriched with different concentrations and combinations of growth regulators (Table 1).
The percentage and time of establishment (days) were assessed.
For the first variable the following specification was taken into account:
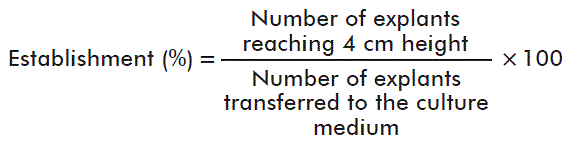
The establishment time was determined when the explants reached a 4-cm height.
Assessment of different growth regulators and combinations for the induction of multiple shoots in vitro
Different concentrations of growth regulators were assessed to induce regeneration of multiple shoots (Table 2).
The number of shoots per explant (up to the third subculture) was assessed every 30 days and the rooting percentage at 90 days. This last variable was assessed according to the formula:

Role of growth regulators in the induction of microbulbils in vitro
Vitroplants from the third subculture of the induction phase of multiple shoots (in vitro multiplication) were used as planting material, which were transferred to the culture medium enriched with sucrose 75 g/L. The treatments used were the following: 1) MS only (absolute control); 2) α-naphthaleneacetic acid (NAA; 0.1 mg/L); 3) isopentenyl adenine (2-iP; 4 mg/L), and 4) NAA (0.1 mg/L) + 2-iP (4 mg/L).
When all leaves were dried, the percentage of bulbification, formation time (days) and transverse diameter of the microbulbils (mm) were assessed.
Bulbification was evaluated according to the following formula:

Acclimatization of in vitro microbulbils
The microbulbils that were induced in vitro with 75 g/L sucrose were cured and stored in Petri dishes at 25 ± 10 ºC and 15 days before planting, they were placed at 4 ºC [6].
The microbulbils were planted at the optimum date for cultivation (October, 2002-2005) and 35 days later they were ready for final field transplantation according to Izquierdo et al. [16]. The combination of substrates used was as follows: 1) 100 % charged zeolite (Litonite); 2) 25 % charged zeolite (Litonite) + 75 % organic matter (Filter cake); 3) 50 % charged zeolite (Litonite) + 50 % organic matter (filter cake); 4) 50 % organic matter (filter cake) + 50 % Eutric compacted red ferralitic soil; 5) 75 % organic matter (filter cake) + 25 % Eutric compacted red ferralitic soil; and 6) 25 % charged zeolite (Litonite) + 50 % organic matter (Filter cake) + 25 % Eutric compacted red ferralitic soil.
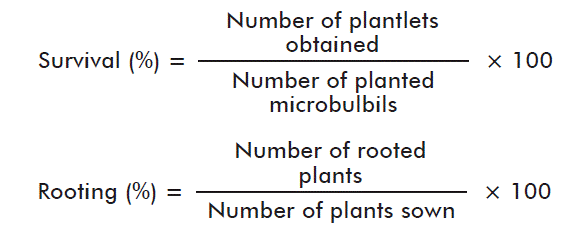
The variables analyzed were: survival (%) and rooting (%), which were assessed by the following equations:
[formula]
Assessment by AFLP of the genetic stability of plantlets obtained in vitro
Garlic plantlets of the best treatments were planted at the end of each of the three subcultures that were performed and from plants from the field. In all cases, the plants had the same physiological age.
The young leaves of each treatment were lyophilized and 100 mg of DNA extracted from the sample by the CTAB method, as modified by Fütterer et al. [17], and stored in 1.5 mL microcentrifuge tubes and at -20 °C until use. The DNA concentration of each sample was adjusted to 30 ng/μL and subjected to PCR analysis by using a TKO 100 Mini-Fluorometer (Hoefer, San Francisco, CA, USA).
The AFLP procedure was carried out according to the methods previously described by Ipek et al. [12]. The following combinations of primers were used to generate the AFLP markers: EAAGG/MCGA, EACGG/MCAT, EACGG/MCTC, EAAGG/MCTC, EAGA/MAGC, ECAC/MAGC and ECAG/MAGC.
For PCR, the following reaction conditions and thermal cycles were used: each 25-mL PCR reaction contained 1.25 U of Taq DNA polymerase (Promega, Madison, WI, USA), with 1× reaction buffer, 0.8 μM of each primer, 200 mM of each dNTP and 60 ng of template DNA. Reactions were incubated at 95 °C for 2 min and subjected to 40 cycles at 94 °C for 30 sec, 58 °C for 1 min and 72 °C for 2 min, with a final 5 min extension step at 72 °C. After 40 cycles, the reactions were carried out at 4 °C. A Perkin Elmer model 9600 thermocycler was used for amplification. Subsequently, the amplification products were denatured at 90 °C for 4 min in an equal volume of formamide buffer and they were immediately placed on ice. Then, the denatured PCR products were separated on 6 % polyacrylamide gels containing 7.5 M urea in 1× Tris-borate (TBE) in 320 μL, 10 % ammonium persulfate and 30 μL of TEMED, and runs were made by applying 3-4 μL of each reaction at 60 W for 2-3 h. In order to visualize the separated fragments, the gels were stained using the Sequence ™ DNA reagent silver staining kit and following the manufacturer’s protocol (Promega). All polymorphic DNA fragments were manually identified on each AFLP autoradiogram and on each of the gels stained for gene-specific markers. The bands were identified as present (1) or absent (0).
Morphoagronomic assessment
Acclimatized plants were taken to the environmental conditions of 35 days (from the best treatment of the acclimatization phase) and were transplanted to the field. After a complete cycle in the field, minibulbils obtained without any agronomic evaluations being made, and they were preserved. All these procedures were carried out following cultural attention and evaluations as recommended by the FAO [6].
In the following campaign, the minibulbils were planted in eutric compacted red ferralitic soil [18], at a distance of 90 + 35 + 35 × 7 cm. Cultural attention was provided according to the Technical Guide for garlic crop production [5]. The size of the plot to be assessed was 1.23 m2.
The variables assessed were: vegetative cycle (days), yield (t/ha), mean bulb mass (g), bulb height (cm), bulb diameter (cm), number of cloves per bulb, mean clove mass (g), clove length (cm) and clove width (cm).
Yield and mean bulb mass were calculated by weight of the total bulbs per plot and for the rest of the variables 10 plants per plot.
RESULTS AND DISCUSSION
Influence of growth regulators on explants establishment in vitro
When evaluating the behavior of the cauline apices in the different culture media, there were significant differences in the percentage of establishment and establishment time thereof (Figure 1). The highest percentage of in vitro establishment was achieved in the culture medium supplemented with NAA (0.1 mg/L) and Kin (0.1 mg/L; 90 %) and the lowest response was for absolute control apices (60.5 %). Similar results were obtained regarding the establishment time of the explants with 18 and 30 days, respectively.
The results obtained with the use of 0.1 mg/L of NAA or IAA and 0.1 mg/L of Kin are similar to those reported in garlic, as for the in vitro response of IAA [19]. On the other hand, Mehta et al. [20] observed that explants inoculated in MS medium containing 6-BAP and Kinetin in the 1-5 mg/L range showed a better establishment and the best response was obtained when those garlic explants were grown with Kinetin (1 mg/L).
Pardo et al. [21] established a protocol for the regeneration of plants from leaf and root segments, in which they determined the effect of auxins and cytokinins combinations, as well as the influence of the explant type and its position in the vitroplant on the induction of calli and their subsequent regeneration to shoots. Explants that were extracted from the apical section of the roots favored callus regeneration, as compared to basal segments of the leaves.
Furthermore, it should be added that the establishment of the explants depends on their origin, the physiological characteristics of the donor plant, the genotype and, to a lesser extent, on the type of biotechnological technique to be used. In this sense, Metwally et al. [22] reported similar results on the isolation, density and viability of protoplasts in two garlic cultivars.
In this phase of in vitro establishment, in different clones or cultivars, it is important to keep the concentrations of auxins and cytokinins very close or similar, so that their response is more effective. In some cases, for this phase it is also recommended NAA, which is a powerful and very stable auxin and provides good results.
Finally, it should be pointed out that for the phase of in vitro establishment, cauline apices (5-10 mm) should be used and transferred to a basal MS medium supplemented with NAA (0.1 mg/L) + Kin (0.1 mg/L).
Assessment of different growth regulators and combinations in the in vitro induction of multiple shoots
The induction of multiple shoots varied according to the type and concentration of the cytokinins with which the culture medium was supplemented, and in all treatments the plantlets rooted, ranging between 40-65 % (Table 3).
In the three subcultures made, the explants emitted a larger number of shoots with the combination NAA (0.1 mg/L) + 2-iP (4 mg/L), which were statistically different from the shoots that were obtained with the rest of the treatments. In the third subculture a decreased number of shoots per explant was observed in all culture media that were used.
Mehta et al. [20] reported that induction of multiple shoots from nodal segments was high when they were obtained in a Kinetin-enriched MS medium (1 mg/L; 3.43 shoots/explant). plantlets were best rooted in this very basal medium, but it was supplemented with IAA (2 mg/L; 3.65 roots/explant). But the results of this work were superior to the previous ones, since a higher multiplication coefficient was obtained and the plantlets rooted in the culture medium itself without the need for transferring them to an additional medium.
In general, the best response was achieved when the culture medium was supplemented with 2-iP (4 mg/L). Furthermore, these shoots grew faster and were more vigorous, which can be considered desirable characteristics for their subsequent use in new in vitro multiplications. The proliferation of explants is lower at low concentrations (1 mg/L).
El-Nasr et al. [11] reported that the Egyptian garlic cultivar ‘Egaseed1’ formed 3 plantlets/explants regardless of the origin of the culture medium, but rooted better in a 50 % MS medium without growth regulators (6 roots/plantlets or 50 % MS with IAA (0.1 mg/L) + Kinetin (1 mg/L; 5.6 roots/plantlet).
As the number of subcultures increases, the multiplication coefficient usually decreases, and in the case of garlic, this may be due to early senescence or dormancy that shoots show from the third subculture on. According to the FAO [6], more than three subcultures are not recommended, since genetic variations can originate in the new regenerants, and it adds that the formation of roots in this crop not only depends on the concentration of growth regulators in the medium but also the in vitro maturity of the cultivars.
In other respects, it is necessary to emphasize that rooting will also depend on the synergic relationship that is established between the growth regulators that are added to the culture medium and the hormones of the plantlets. Izquierdo [23] reported that the MS medium contains relatively high concentrations of nitrogen and potassium compared to other culture media and, therefore, it met the nutritional requirements of different plant species. Additionally, the differences obtained in the in vitro response in this phase were due to the influence exerted by the genotype, since each responds differently [23].
With none of the combinations of growth regulators studied, callus formation occurred, which is considered a desired characteristic, since it may induce genetic or cytogenetic changes that undermine the stability of the material to be propagated. This could also be due to the fact that the content of endogenous auxins was adequate.
For all of the above, it is proposed to inoculate the explants in an MS medium supplemented with NAA (0.1 mg/L) + 2-iP (4 mg/L), where vitroplants also form roots without having to be transferred to an additional culture medium.
Role of growth regulators in the induction of microbulbils in vitro
In table 4, the results that relate to the influence of growth regulators regarding in vitro bulbification can be seen. As observed, there are significant differences between treatments. The best results were obtained when microbulbils were induced in vitro in an MS medium without growth regulators (Treatment 1, 99.58 %). Their time of induction behaved inversely to the bulbification percentage, ranged between 40- 55 days; the best results were obtained by microbulbils that were induced in vitro with treatment 1 (40 days).
In vitro microbulbification occurs due to the high concentration of sucrose in the culture medium, which causes an increase in water stress in the plantlets by an increase in the osmotic potential. Thus, the in vitro induction of microbulbils varies for each genotype and it is generally induced from six weeks on (45 days). This phenomenon depends on the culture medium and the physiological characteristics of the plant from which the explant and the cultivar were extracted [24].
Sucrose is used as energy source during the formation of in vitro garlic microbulbils, and high concentrations induce the synthesis of some storage- and osmotic stress-related proteins, as well as reserve amino acids, which influence the formation of these bulbils [25-28]. Besides, Pardo et al. [13] stated that the reduction of the MS medium at 75 and 50 %, both supplemented with sucrose, allowed the conservation of microbulbils stable or retaining their type for a period of 210 days; while irradiation with 8 or 10 krad, led to the induction of genetic changes or variations in garlic microbulbils.
There were also significant differences between treatments attending to sectional diameter and the mean mass of microbulbils (Table 4). The sectional diameter ranged between 3.85-5.35 mm and the mean mass of microbulbils ranged 842.65-1455.20 mg. The best results were reached by the microbulbils generated with Treatment 1. There is a close relationship between the sectional diameter and the mean mass of the bulbils obtained in vitro; microbulbils larger than 3 mm in diameter can reach the following year an 85 % field establishment. According to FAO [6], among these parameters there is a very high exponential correlation which makes its use indistinct for classifying them. It adds that microbulbils with diameter equal to or greater than 4 mm, when taken to field can form bulbils between 15-25 Mm and that from the microbulbil to the bulbil for consumption, an increase in size occurs, due to the physiological maturation of the clones.
We consider that it is not necessary for the microbulbification phase to supplement culture media with growth regulators (auxins and/or cytokinins) or other substances that act in a similar way to these regulators, since in vitro bulbification appears to be related with the concentration of sucrose added to the culture medium, as well as the reserves and endogenous mobilization of hormones that are added to the culture medium in the establishment and in vitro multiplication stages, which influence the induction of this in vitro bulbification process.
Acclimatization of in vitro microbulbils
Microbulbils showed a good behavior under the environmental conditions. As shown in Table 5, there were significant differences between the treatments attending to survival, height and number of leaves per plant in the different substrates that were used. Survival of microbulbils that were induced in vitro was high, ranging between 92.97-99.40 % and the number of leaves per plant between 15.08-16.03. The best results for both, survival and height were for plants that were obtained with substrate variant 2 (0.25 % Zeolite + 75 % organic matter). Izquierdo et al. [16] reported results similar to those of this work in different garlic clones (four ‘Criollo’ and two ‘Vietnamese’ clones).
In correspondence with the above considerations, the results showed that the use of zeolite mixed with organic matter favored the survival of microbulbils obtained in vitro, what seems to be very closely related to the chemical and physical properties of this mineral.
The improvement generally obtained in the development of plants by the use of the Zeolite + organic matter mixture could be associated with the retention by the zeolite of the nutrients that are released in the filter cake mineralization process, so their loss is avoided and, consequently, increases their availability for plants. According to Metwally et al. [29] when the bulbs obtained in vitro are transferred to soil, they reach a 60 % survival in the first three months of cultivation. They add that their size determines the quality of the plants to be grown in the field and the time period they require to obtain commercial size.
From the results, we can state that the acclimatization of the microbulbils should be done in polypropylene trays containing a substrate made up of 25 % Zeolite + 75 % organic matter. The plants are considered to be suitable for final field transplantation at 35 days, having more than three leaves, 15 cm or more in height and more than 98 % of rooting.
Assessment of the genetic stability of plantlets obtained in vitro by AFLP
Results of the AFLP technique showed a total of 130 clearly separated bands, with an average of 175.71 bands per each combination of primers being analyzed. All the bands quantified were monomorphic (100 %), so the phylogenetic distance was 0. The same number of bands were detected with each primer set on a parental plant collected from the field and in those subjected to three different culture treatments (unsupplemented MS medium, absolute control; MS medium plus NAA (0.1 mg/L) plus Kin (8 mg/L); MS medium supplemented with NAA (0.1 mg/L) plus 2-iP (4 mg/L)) in three subcultures, respectively. Bands corresponding to each primer sets were distrib-uted as follows: primers EAAGG/MCGA (18 bands) EACGG/MCAT (21), EACGG/MCTC (19), EAAGG/ MCTC (14), EAGA/MAGC (10), ECAC/MAGC (15) and ECAG/MAGC (26). This analysis indicates that there were no differences at molecular level between vitroplants regenerated in the different culture media, in the three subcultures that were grown and the stock from the field, for the combinations of primers that were analyzed. Otherwise, this molecular evidence is not conclusive because AFLP markers amplify certain sites in the genome, in spite of these molecular studies were corroborated with the morphological results under field conditions.
Ipek et al. [30], reported that the AFLP assay with three combinations of primers: EACGG/MCAT, EACGG/MCTC and EAAGG/MCGA obtained a high polymorphism in the 22 garlic clones they studied. However, they did not report the total of monomorphic or specific bands of cultivars. Other less polymorphic primers were also used [31], but the authors reported that these primers had between 4 and 30 common bands, between 8 and 17 specific bands, between 10 and 17 polymorphic bands, for a total of bands ranging from 25 to 50. Our results are similar to those obtained by the previous authors, but different from those of Ipek et al. [30]. They used these primers for the characterization of cultivars, and we used them instead to determine the genetic stability of a clone of Cuban garlic obtained by selection, virus-cleaned and micropropagated, in which this molecular technique was used for the first time.
Taking into account that garlic has an asexual reproduction system and, therefore, does not perform the genetic recombination process; a 43% polymorphism is considered high [30]. Nevertheless, it is lower than for other species such as common bean (Phaseolus vulgaris L.), which has 53.4 % divergence [32]. A higher percentage of polymorphism is understandable in species with sexual reproduction systems, as it allows a higher recombination level in these plants [33].
It was also reported that 16 primers of SSR markers were used to determine genetic variability within germplasm collections of the Allium genus, for use in genetic breeding programs and obtaining new cultivars of high nutritional value [1].
According to Cheng et al. [34], the number of chromosomes from in vitro root apices originating from calli, previously treated with trifluralin produced plants with different ploidies: mixoploids, chimeras, diploids and tretaploids, which depended on the concentration of the product and the treatment used. On the other hand, Scotton et al. [35] obtained 2.45 shoots from calli per explant with the culture medium: MS + 8.8 mM BAP + 0.1 mM NAA. However, when it is desired to obtain a micropropagation protocol, 2,4-D should not be used to induce calli or indirect organogenesis under in vitro conditions since it generally induces genetic variability in the regenerants obtained.
In a comparative analysis of the genetic diversity of olive genotypes with AFLP, RAPD and SSR markers, it was found that AFLPs were the most effective marker system, although the expected heterozygosity was the lowest [36].
Ipek et al. [37] reported 130 polymorphic DNA fragments amplified from 26 EST-SSR markers, the number of polymorphic alleles per SSR marker ranging 2-13 with an average of 5 alleles. They added that the heterozygosity observed and the information content of the polymorphism (PIC) of SSR markers ranged from 0.23 to 0.88, and from 0.20 to 0.87, respectively. So, they concluded that EST-SSR markers can be used in genetic studies, such as genetic mapping, map clustering, genetic diversity and in genome comparison studies of Allium species.
It should be added that in previous researches conducted in Cuba with different garlic clones (four ‘Criollo’ and two ‘Vietnamese’ clones), it was verified by karyotype studies on vitroplants from different subcultures and field plants that the number of chromosomes remained constant (2n = 2x = 16). Likewise, eight RAPD primers (OPA-01, OPAB-04, OPAB-18, OPC-09, OPC-12, OPD-01, OPD-07 and OPO-01) were assessed, generating between 2-10 bands, all of them monomorphic. Different isoenzyme systems (carbonic anhydrase, esterases, peroxidases, polyphenoloxidase, malate dehydrogenase and isocitrate dehydrogenase) were also studied and there was 100 % monomorphism. Nevertheless, the joint study of these techniques and the morphoagronomic assessments made it possible to differentiate the ‘Criollo’ and ‘Vietnamese’ groups, and also the different ‘Criollo’ clones, ‘Criollo-9’ among them.
Izquierdo et al. [38] reported that there was no genetic variability in the ‘FHIA-18’ (AAAB) banana clone that had been propagated in vitro and acclimatized using different concentrations of traditional growth regulators (auxins and/or cytokinins) and nontraditional (Pectimorf® and Biobras-6®). They used different techniques, such as cytogenetics, isoenzymes (four systems) and RAPD (four primers), in the last two cases the most polymorphic reported so far for the Musa spp. genus or specifically for that banana clone.
Taking into consideration the results presented so far, the novelty of this work is related to the establishment of a methodology for the micropropagation of the ‘Criollo-9’ garlic clone, and the use of different combinations of AFLP primers, which did not generate genetic variability. Therefore, the genetic stability of the regenerants obtained, at least with the primers used in this study, was confirmed.
Morphoagronomic assessment of plants propagated in vitro
All the plants survived under field conditions; the vegetative cycle was 135 days, without significant differences between the treatments. Nevertheless, there were significant differences regarding the yield of the plants (Figure 2).
The yields obtained were higher than those reported in the Technical Guide for garlic crop production [5].
It was shown that the highest yields were obtained by the genotypes of microbulbils obtained with the highest concentration of sucrose (75 g/L).
Concerning the number of leaves per plant and the height of the foliage, they are in correspondence with the yield obtained. These results coincide with those of Izquierdo [23], who states that yield depends on the leaf area the plant develops during the vegetative stage and on the time it remains physiologically active. Thus, there is a greater translocation of assimilates from the leaves to the bulb, which is a storage organ and, therefore, it provides a higher yield.
FAO [6] reported that the optimum yield in garlic generated by in vitro cultivation occurs in the fourth year and then begins to decline. Importantly, our results do not coincide with the previous ones, since at the fourth year this genotype continued to increase its yield (data not shown). FAOSTAT [4] reported yields of 11.78 t/ha for this crop, yields lower than the ones obtained in this work, since this clone is of high biological quality.
Both, the characteristics of the bulbs and the cloves showed significant differences between treatments (Table 6). In both cases, the best overall results were for the bulbs and cloves obtained with Treatment 2 (25 % Zeolite + 75 % organic matter).
These results indicated that there is a close relationship between mass, height and diameter of the bulb with yield, which agrees with Izquierdo [23]. There is also a relationship between yield and clove length, so the results of this work are similar to those of Gómez et al. [9].
Regarding foliage, it was green in all cases; the external and internal color of the bulbs and cloves was white. These results coincide with those of Gómez et al. [9] and Izquierdo [23]. Additionally, the plants did not show symptoms of phytopathogenic diseases and the bulbs showed a good behavior during the storage phase before white rot (Sclerotium cepivorum Berk), so post-harvest losses diminished in this phase due to decreased physiological mass and seed emptying (25-30 %), since they had a dry matter content of 38-40 % at six months (data not shown).
The culinary and medicinal properties of this crop are associated with its aromatic components and its reserve carbohydrates (fructans), which generally constitute more than 70 % of the dry matter of the clove. The commercial quality of the bulb is given by its size, shape and absence of defects, the largest, the most regular and those without visual defects being better accepted. These factors are determined by the genotype, the environmental conditions of temperature and photoperiod, as well as the sowing date, fertilization rate and tillage. All of them interact and generate physiological responses and a quality of the finished product [39, 40].
Garlic requires low temperatures during its development in order to generate good quality bulbs, although it is not known exactly its requirements or the physiological effects of this factor on the development of the plant.
As previously stated, the results of the molecular studies were corroborated under field conditions, since the crop yield increased by more than 12 t/ha, being a material of high biological quality and basic seed can be obtained by this micropropagation technique for the garlic ‘Criollo-9’ clone.
CONCLUSIONS
A methodology was obtained for the micropropagation of garlic (Allium sativum L.) ‘Criollo-9’ clone, which did not induce genetic variability in the regenerants obtained. It was verified by using different AFLP primers, showing the same number of bands between the stock and the plantlets obtained in vitro in the three subcultures grown. Furthermore, with this methodology, efficient culture media and substrates are proposed, which provided yields of more than 12 t/ha under field conditions.
It is also recommended to assess new combinations of AFLP markers and other molecular markers, such as SSR, to corroborate the genetic stability of the garlic (Allium sativum L.) ‘Criollo-9’ clone and to demonstrate that applying this micropropagation technique in this crop is feasible.
ACKNOWLEDGEMENTS
To the Ministry of Foreign Relations (SRE, Spanish acronym) of the Government of Mexico for granting a Postdoctoral Excellence Scholarship for Foreigners, which allowed the writing of this article during the stay made throughout the project: “Use of Bioactive Products in Propagation through Tissue Culture and Acclimatization of Plants of Agricultural Interest”.
REFERENCES
1. Cunha CP, Hoogerheide ES, Zucchi MI, Monteiro M, Pinheiro JB. New microsatellite markers for garlic, Allium sativum (Alliaceae). Am J Bot. 2012;99(1):e17-9.
2. Arzanlou M, Bohlooli S. Introducing of green garlic plant as a new source of allicin. Food Chem. 2010;120:178-83.
3. Rana SV, Pal R, Vaiphei K, Sharma SK, Ola RP. Garlic in health and disease. Nutr Res Rev. 2011;24(1):60-71.
4. FAOSTAT. Food and Agriculture Organization of the United Nations, FAO. 2014 [cited 2015 Dec 30]. Available from: http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID.
5. Marrero A, Hernández A, Caballero R, Casanova A, Jiménez J, Iglesias I, et al. Guía técnica para la producción del cultivo del ajo. La Habana: MINAG. Instituto de Investigaciones Hortícolas “Liliana Dimitrova”. Asociación Cubana de Técnicos Agrícolas y Forestales; 2009.
6. FAO. Intercambio y propagación de germoplasma de ajo a través de microbulbillos. Córdoba: Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba; 1991.
7. Keller ERJ, Senula A. Micropropagation and cryopreservation of garlic (Allium sativum L.). In: Lambardi M, et al., eds. Protocols for micropropagation of selected economically-important horticultural plants. New York: Springer; 2013. p. 353-68.
8. Gervini de Menezes FO. Garlic in vitro culture for clonal recovering virus-free plants. Rev Ciênc Agrovet Lages. 2011;10(2):158-67.
9. Gómez O, Savón JR, Espinosa J, Hernández T. Estudio de la variabilidad encontrada en clones de ajo en la provincia de La Habana. Agrot Cuba. 1991;23(1-2):1-4.
10. Rai MK, Shekhawat NS, Harish P, Gupta AK, Phulwaria M, Ram K, et al. The role of abscisic acid in plant tissue culture - a review on the recent progress. Plant Cell Tiss Org Cult. 2011;106:179-90.
11. El-Nasr SH, Ahmed KZ, Moustafa YMM, Ezzat AS. Growth and cytogenetical properties of micro-propagated and successfully acclimatized garlic (Allium sativum L.) clones with a modified shoot tip culture protocol. J Hort Sci Ornam Plants. 2011;3(2):115-29.
12. Ipek M, Ipek A, Simon PW. Comparison of AFLPs, RAPD markers, and isozymes for diversity assessment of garlic and detection of putative duplicates in germplasm collections. J Am Soc Hort Sci. 2003;128:246-52.
13. Pardo A, Hernández A, Méndez N, Alvarado G. Análisis genético, mediante marcadores RAPD, de microbulbos de ajo conservados e irradiados in vitro. Bioagro. 2015;27(3):143-50.
14. Jo M, Ham I, Moe K, Kwon S, Lu F, Park Y, et al. Classification of genetic variation in garlic (Allium sativum L.) using SSR markers. Austr J Crop Sci. 2012;6 (4):625-31.
15. Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473-97.
16. Izquierdo H, Quiñones Y, Disotuar R, Pedroso D. Evaluación de diferentes sustratos en la aclimatización de vitroplantas y microbulbillos de ajo (Allium sativum L.). Cult Trop. 2002;23(3):63-9.
17. Fütterer J, Gisel A, Iglesias V, Kloti A, Kost B, Mittelsten-Scheid O, et al. Standard molecular techniques for the analysis of transgenic plants. In: Potrykus I, Spangenberg G. (Eds.), Gene Transfer to Plants. New York: Springer-Verlag; 1995; p. 215-8.
18. Hernández A, Ascanio MO, Morales M, Cabrera A. Correlación de la nueva versión de clasificación genética de los suelos de Cuba con las clasificaciones internacionales y nacionales: una herramienta útil para la investigación, docencia y producción agropecuaria. La Habana: Instituto Nacional de Ciencias Agrícolas (INCA); 2005. p. 18-59.
19. Conci V, Moriconi DN, Nome SF. Cultivo de meristemas apicales de seis tipos clonales de ajo (Allium sativum L.). Phyton. 2005;46(2):187-94.
20. Mehta J, Sharma A, Sharma N, Megwal S, Sharma G, Gehlot P, et al. An improved method for callus culture and in vitro propagation of garlic (Allium sativum L.). Int J Pure App Biosci. 2013;1(1):1-6.
21. Pardo A, Luna F, Hernández, N. Regeneración in vitro de Allium sativum L. a partir de segmentos de hojas y raíces. Bioagro. 2011;23(3):207-14.
22. Metwally EI, El-Denary ME, Dewir YH. Influences of explant types and enzyme incubation on isolated protoplast density and viability in two garlic cultivars. Pak J Bot. 2014;46:673-7.
23. Izquierdo H. Propagación in vitro de genotipos cubanos de ajo (Allium sativum L.). Tesis de Maestría. La Habana: Facultad de Biología. Universidad de La Habana; 2000.
24. Mujica H, Mogollón N. Bulbificación in vitro del ajo (Allium sativum L.) con adición de citocininas y sacarosa en el medio de cultivo. Bioagro. 2004;16(1):55-60.
25. Rai MK, Shekhawat NS, Gupta AK, Phulwaria M, Ram K, Jaiswal U. The role of abscisic acid in plant tissue culture - a review on the recent progress. Plant Cell Tiss Org Cult. 2011;106:179-90.
26. Dixit V, Rai, SP, Chaudhary BR. Allium sativum: Four-step approach to efficient micropropagation. Int J Innov Biol Res. 2013;2(1):6-14.
27. Hachez C, Besserer A, Chevalier AS, Chaumont F. Insights into plant plasma membrane aquaporin trafficking. Trends Plant Sci. 2013;18(6):344-52.
28. Pereyra M, Quiriban A. Las proteínas en la tolerancia al estrés hídrico en plantas. Semiárida. 2014;24(1):55-67.
29. Metwally EI, El-Denary ME, Omar AMK, Naidoo Y, Dewir YH. Bulb and vegetative characteristcs of garlic (Allium sativum L.) from in vitro culture through acclimatization and field production. Afr J Agric Res. 2012;7:5792-5.
30. Ipek M, Ipek A, Simon PW. Molecular characterization of Kastamonu garlic: An economically important garlic clone in Turkey. Sci Hort. 2008;115:203-8.
31. Lee MK, Lim YP, Bang JW. Genetic analysis of garlic (Allium sativum L.). Kor J Genet. 2002;24(1):75-81.
32. Kumar V, Sharma S, Kero S, Sharma S. Assessment of genetic diversity in common bean (Phaseolus vulgaris L.) germplasm using amplified fragment length polymorphism (AFLP). Sci Hortic. 2008;116:138-43.
33. Morales RGF, Resende JTV, Resende FV, De la torre CA, Figueiredo AST, Da-Silva PR. Genetic divergence among Brazilian garlic cultivars based on morphological characters and AFLP markers. Genet Mol Res. 2013;12(1):270-81.
34. Cheng ZH, Zhou XJ, Khan MA, Su L, Meng HW. In vitro induction of tetraploid garlic with trifluralin. Genet Mol Res. 2012;11(3): 2620-8.
35. Scotton DC, Benedito VA, Molfetta JB, Rodrigues BIFP, Tulmann-Neto A, Figueira A. Response of root explants to in vitro cultivation of marketable garlic cultivars. Hortic Bras. 2013;31:80-5.
36. Ipek M, Seker M, Ipek A, Gul MK. Identification of molecular markers associated with fruit traits in olive and assessment of olive core collection with AFLP markers and fruit traits. Genet Mol Res. 2015;14(1):2762-74.
37. Ipek M, Sahin N, Ipek A, Cansev A, Simon PW. Development and validation of new SSR markers from expressed regions in the garlic genome. Sci Agric. 2015;72(1):41-6.
38. Izquierdo H, González MC, Núñez MC. Genetic stability of micropropagated banana plants (Musa spp.) with non-traditional growth regulators. Biotecnol Apl. 2014;31(1):23-7.
39. Mathew D, Forer Y, Rabinowitch HD, Kamenetsky R. Effect of long period on the reproductive and bulbing processes in garlic (Allium sativum L.) genotypes. Environ Experim Bot. 2011;71:166-73.
40. Khar A, Banerjee K, Jadhav MR, Lawande KE. Evaluation of garlic ecotypes for allicin and other allyl thiosulphinates. Food Chem. 2011;128:988-96.
Received in May, 2016.
Accepted in December, 2016.
Humberto Izquierdo-Oviedo. Instituto Nacional de Ciencias Agrícolas, INCA. Carretera a Tapaste, Km. 3 ½, San José de las Lajas, CP 32700, Mayabeque, Cuba. E-mail: hioviedo@inca.edu.cu , hoviedo1966@gmail.com.














