Meu SciELO
Serviços Personalizados
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Biotecnología Aplicada
versão On-line ISSN 1027-2852
Biotecnol Apl vol.34 no.3 La Habana july.-set. 2017
RESEARCH
Assessment of the chromatographic resins Cellufine MAX Q-hv and Capto Q for r-HBsAg purification
Evaluación de las resinas cromatográficas Cellufine MAX Q-hv y Capto Q para la purificación del r-HBsAg
Pedro Domínguez Aldás1, Lourdes Zumalacárregui de Cárdenas2, Abel Caballero1
1 Centro de Ingeniería Genética y Biotecnología, Ave 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
2 Facultad de Ingeniería Química, Universidad Tecnológica de La Habana José Antonio Echeverría. Calle 114 No 11901 entre Ciclovía y Rotonda, CP 19390, La Habana, Cuba.
ABSTRACT
A thermodynamic and kinetic understanding of chromatographic separation is required for the design of the chromatographic process’ operating system. In this work, the anion exchange chromatographic resins Cellufine MAX Q-hv from JNC Corporation (Japan) and Capto Q from GE Healthcare Life Science (USA) were evaluated for the purification process of the recombinant hepatitis B surface antigen (r-HBsAg), as active pharmaceutical ingredient. The highest r-HBsAg recoveries were obtained with Capto Q resin (87.16 ± 1.34 %). This value was statistically different (p < 0.05) from that of Cellufine MAX Q-hv resin and statistically similar (p > 0.05) to that of TMAE anion resin (Merck), currently in use for the industrial production process of the HeberBiovac HB® vaccine. Cellufine MAX Q-hv and Capto Q resins were assessed kinetically and thermodynamically, and their the adsorption behavior of r-HBsAg were predicted by Langmuir isotherms with a good adjustment (R2 > 90 %). The Capto Q resin had an association constant 3.89 times higher than the Cellufine MAX Q-hv resin. The maximum adsorption capacities of both resins were similar, with values between 1.5 and 1.8 mg of r-HBsAg/mL of gel. The dynamic adsorption capacity of Cellufine MAX Q-hv resin (1.5 mg r-HBsAg/mL of resin) was higher than that for Capto Q (0.9 mg r-HBsAg/mL of resin), with more than 40 % of the chromatographic bed of both resins used is inefficiently. Both resins can be introduced for the industrial purification of the r-HBsAg, attending to process specifications.
Keywords: Anion exchange chromatography, adsorption isotherms, breakthrough curves, r-HBsAg.
RESUMEN
Para el diseño de las condiciones de operación del proceso cromatográfico se requiere una comprensión termodinámica y cinética de la separación cromatográfica. En este trabajo, se evaluaron dos resinas cromatográficas de intercambio aniónico en el proceso de purificación del ingrediente farmacéutico activo r-HBsAg: Cellufine MAX Q-hv de la firma JNC Corporation (Japón) y Capto Q de la firma GE Healthcare (EEUU). Los mayores recobrados de r-HBsAg se obtuvieron con la resina Capto Q (87.16 ± 1.34 %). Este valor fue estadísticamente diferente (p < 0.05) con respecto a la otra resina y similar estadísticamente (p > 0.05) al alcanzado con la resina aniónica actualmente utilizada (TMAE, Merck) en el proceso industrial. Se evaluaron cinética y termodinámicamente ambas resinas. La isoterma de Langmuir predijo, con un buen ajuste (R2 > 90 %), el comportamiento de la adsorción del r-HBsAg en las resinas ensayadas. La resina Capto Q presentó una constante de asociación 3.89 veces superior que la resina de Cellufine MAX Q-hv. Las capacidades máximas de adsorción para las resinas fueron similares, con valores entre 1.5 y 1.8 mg de r-HBsAg/mL de gel. La capacidad dinámica de adsorción de la resina Cellufine MAX Q-hv (1.5 mg r-HBsAg/mL de resina) fue mayor que la obtenida para la resina Capto Q (0.9 mg de resina r-HBsAg/mL de resina) y más del 40 % del lecho cromatográfico para ambas resinas no se está utilizando eficientemente. Ambas resinas se pueden utilizar para la purificación del r-HBsAg.
Palabras clave: Cromatografía de intercambio aniónico, isotermas de adsorción, curvas de ruptura, r-HBsAg.
INTRODUCTION
Hepatitis B is a viral infection of the liver that can lead to both an acute setting and a chronic illness. The virus is transmitted by contact with blood or other body fluids from an infected person and can lead to liver failure, cirrhosis or liver cancer. This disease is one of the main health problems worldwide, with an estimated prevalence of two billion people and about 780 000 deaths each year. In this setting, vaccination is the main pillar for the prevention of this disease [1, 2].
In 1989, the recombinant Cuban anti-hepatitis B vaccine Heberbiovac HB® was obtained at the Center for Genetic Engineering and Biotechnology (CIGB), using a recombinant strain of Pichia pastoris yeast. The active pharmaceutical ingredient (API) for the hepatitis B vaccine, consisting on the recombinant hepatitis B virus surface antigen (rHBsAg), has been manufactured industrially since 1990 and the vaccine included in the national immunization program as the Heberbiovac HB® vaccine. This vaccine has proven effective for mass vaccination and has allowed the prevention and has supported the gradual eradication of HBV prevalence in Cuba [3, 4]. Moreover, the use of rHBsAg as API has been diversified, with its inclusion as part of the vaccine HeberNasvac® [5, 6] to treat chronically infected HBV patients, and the pentavalent vaccine Heberpenta® against five different diseases in children [7].
For their production, chromatographic systems and their performance are key elements in demonstrating the reproducibility of the industrial process. The increasing demand for pharmaceutical products requires reproducible and consistent fractionation mechanisms providing them high levels of purity and stability in a short time, at the lowest possible cost and with the required quality [8, 9]. In this line, protein purification schedules are designed to remove impurities from the final product, with as few chromatographic methods and steps as possible to guarantee the best recovery with throughput operability, and with optimal process performance at any scale [8, 10, 11]. One of the most common fractionation and purification techniques is Ion Exchange Chromatography, being applied to proteins, polypeptides, nucleic acids, polynucleotides and other biomolecules. It has a high resolution capacity, high load, simplicity and fine control method during the purification process [12, 13].
Although affordable levels of process consistency and effectiveness have been attained and are implemented at different production scales, process optimization could be possible due to the availability of new resins with adequate properties for better process performance. In this sense, new types of resins are being generated, with adequate physico-chemical properties and complying with the most stringent regulatory requirements for the industry. Moreover, a thermodynamic and kinetic understanding of chromatographic separation and an adequate selection of the chromatographic matrices is required for the design of the operating conditions of the chromatographic process [14, 15]. There has been reported that up to 47 % of existing biopharmaceutical companies argued in favor of using new chromatographic matrices with high adsorption capacities to eliminate bottlenecks in purification processes [16]. As a result, the suppliers of chromatographic matrices focus on the development of adsorbents with a structure that guarantees better properties associated with mass transfer.
Therefore, in this work, we studied the thermodynamic and kinetic assessment of two of those new chromatographic resins, Cellufine MAX Q-hv (JNC Corporation, Japan) and Capto Q (General Electric Healthcare Life Science, USA), for the purification of the rHBsAg, in comparison with currently used TMAE resin (Merck, Germany).
MATERIALS AND METHODS
Materials and reagents
All materials and solutions were analytical grade, complying with standard specifications for purification processes in the biopharmaceutical industry. Hydrochloric acid, sodium chloride, sodium deoxycholate, ethylenediaminetetraacetic acid, sodium hydroxide and hydroxymethylaminomethane were purchased from Merck, Germany, and potassium thiocyanate from Rutgers, Germany. Ethanol was supplied by Cuba Ron S.A and water for injection was produced at the CIGB manufacturing facility (Havana, Cuba).
Chromatographic resins
Chromatographic resins Cellufine MAX Q-hv (JNC Corporation, Japan) and Capto Q (General Electric Healthcare Life Science, USA) were studied, following manufacturers’ specifications as strong anion exchange matrices. Particles were spherical, containing quaternary aminoethyl functional group (Q type), with a particle diameters in the range 0.04 -0.13 mm for Cellufine MAX Q-hv and 0.09 mm for Capto Q, as reported by the manufacturers. Their reported dynamic capacities were 135 mg/mL (Cellufine MAX Q-hv) and 100 mg/mL (Capto Q), as determined for the adsorption of Bovine Serum Albumin (BSA). Fragtogel EMD TMAE resin (Merck, Germany) was used for comparison, as the matrix currently used for the industrial purification process of the rHBsAg antigen; TMAE particles were spherical, with a particle diameter in the range 0.04-0.09 mm.
The sanitizing solutions used are in compliance with SOP 2.02.600.91 [18].
The equipment used in the chromatographic runs were: glass chromatographic column for XK-26/40 bioprocesses of 2.6 cm of internal diameter and 40 cm of height (GE Healthcare Life Science, USA), microcomputer with data acquisition program (Biochrom, CIGB, Version 3.0), piston pump (La Chrom, L-7150, Merck, Germany), UV detector and optical unit UV-1 type (GE Healthcare Life Science, USA).
Sampling and determination of rHBsAg concentration
Samples of protein material were taken from the industrial manufacturing process to perform the assessments, and the different solutions were prepared following the standard current procedure SOP 2.02.603.91 [14]. rHBsAg was quantitated in solution by absorbance measurements at 280 nm, using a molar extinction coefficient (εM) of 5, in a 1-cm path-length optical cuvette according to SOP 2.02.622.91 [19].
Working procedure for chromatographic runs
Chromatographic matrices were evaluated for rHBsAg purification, following an operational similarity criterion of keeping constant the residence time (rt) of the rHBsAg into contact with the packed bed, from the experimental to the industrial scale processes [11, 15, 20].
Operational parameters for the experimental scale were: 5.6 cm/h linear flow, 41 cm3/h volumetric flow, 5.6 cm height of packed resin, 30 mL of packed resin and 0.7 h (42 min) rt of the adsorbate in the adsorbent. The scale ratio was 853. The experimental scale represented 0.12 % of the gel volume and volumetric flow of the industrial scale.
The experimental assessment of the chromatographic resins involved six steps: column packing, column equilibration, application of protein material, resin wash, elution and column regeneration. The main properties of each step were:
Packing
The new chromatographic resins were washed with five column volumes (CVs) of water for injection and further packed up with the packaging solution designed for each resin (10 mmol/L NaCl for Cellufine MAX Q-hv and 1 mol/L NaCl for Capto Q) at a flow rate of 0.06 L/h. Subsequently, the matrix was loaded with two CVs of 0.1 mol/L HCl, five CVs of water for injection, two CVs of NaOH 0.2 mol/L and five CVs of water for injection were applied.
Column equilibration
Three CVs of each equilibration solution were applied in two subsequent equilibration steps: 1) 200 mmol/L Tris-HCl, EDTA 30 mmol/L, pH 7.2; and 2) 200 mmol/L Tris-HCl, 3 mmol/L, pH 7.2 EDTA.
Application of protein material
The rHBsAg protein was applied at a protein mass (mg) per resin volume (mL) ration in the range 0.9-1.3.
Resin wash
After the application of the protein material to the packed bed was completed, one CV of the second equilibration solution was added, followed by three CVs of the wash solution (20 mmol/L Tris-HCl, 3 mmol/L EDTA, 50 mmol/L NaCl, pH 7.2).
Elution
The eluate was collected since the start of chromatogram signal increase until the signal reached 5 % of the peak height from baseline. For this step, the elution solution was used with the following composition: Tris-HCl 20 mmol/L, 3 mmol/L EDTA, 600 mmol/L NaCl, 0.05 % sodium deoxycholate; pH 7.2.
Resin regeneration
Two CVs of the regeneration solution corresponding to each resin were applied for this step (20 mmol/L Tris- HCl, 3 mol/L KSCN, pH 7.6, for Cellufine Max Q-hv; 20 mmol/L Tris, 3 mmol/L EDTA, 2 mol/L NaCl, pH 7.2, for Capto Q). Chromatographic runs were performed at a 0.04 L/h flow rate of. The Biocrom program (CIGB, Version 3.0) was used for recording and acquiring the UV detector’s signal, at a wavelength of 280 nm. All runs were performed at room temperature (22 ± 1 °C) and in triplicate for each resin.
The recovery of the anion exchange chromatography was determined by using the following formula:
![]()
Where:
RIE: recovery from the ion exchange step.
rHBsAgIE: mass of rHBsAg in the eluate of the ion exchange chromatography.
rHBsAgInput: mass of rHBsAg applied to the ion exchange chromatography.
Determination of adsorption kinetics
The adsorption kinetics of the Cellufine MAX Q-hv and Capto Q chromatographic resins was determined by considering 2 mg of rHBsAg/mL of gel to be adsorbed. For this, 25 mL of each resin, previously equilibrated with a solution from the second equilibration step, were added to a sample of 251 mL of protein material. The mixing process was performed under agitation with a magnetic stirrer at a speed to keep the chromatographic resin suspended while preventing the formation of a vortex on the liquid surface. Sampling started once the resin was completely added, with 1.5 mL-samples taken from the suspension at predefined time intervals (Table 1) until the concentration of the adsorbent in the liquid phase remained constant.
Afterwards, samples were centrifuged at 9390 × g for 1 min. The supernatant was used to determine rHBsAg concentration by optical density (OD). The experiments were performed in duplicate at a temperature of 22 ± 1 °C. The program CurvaExpert (version 1.3) was used to adjust the curves.
Determination of adsorption isotherm
Adsorption isotherms were constructed for evaluating the adsorptive capacity of the Cellufine MAX Q-hv and Capto Q resins. For this purpose, 13 reaction vials of 1.5 mL were prepared, containing 0.4 mL of the chromatographic resin previously equilibrated with the solution of the second equilibration step and a given calculated volume of the protein material. The initial rHBsAg concentration ranged from 0.24 to 2.3 mg per milliliter of gel (0.8, 1.2, 1.6, 2.0, 2.4, 2.8, 3.2, 3.6, 4.0, 4.4, 6.0, 7.0 and 8.0 mg rHBsAg/mL gel). Vials were stirred in the bascule stirrer, for the minimum contact time as previously determined in the adsorption kinetics experiment. Afterwards, the reaction vials were centrifuged at 9391 × g for 1 min. The supernatant was then collected for determining the rHBsAg concentration by OD.
The final concentration of the adsorbate was set as the solute concentration in the liquid phase in equilibrium. The solute concentration in equilibrium in the solid phase was determined by mass balance between the solid phase and the liquid phase. The Statgraphics centurion XVI.II program was used to represent the adsorption behavior. These experiments were performed in duplicate at 22 ± 1 °C.
Determination of gel breakthrough curve
The protein material was applied to the chromatographic column packed either with Cellufine MAX Q-hv or Capto Q resins until rHBsAg concentrations of the starting material and the column effluent were the same. It was established from the chart obtained that the breakthrough time corresponded to an output concentration/input concentration ratio of 0.05. Likewise, saturation time was set as the time required for the output concentration of the chromatographic bed to equal the input concentration.
Statistical analysis
The statistical analysis was performed for a single factor using the Statgraphics Centurion XVI.II program. Significance analysis was done by running an ANOVA F-test for a confidence level of 95 %, with previous analysis of the normal distribution of the response variable.
RESULTS AND DISCUSSION
Assessment of the anion exchange step for Cellufine MAX Q-hv, Capto Q and TMAE chromatographic resins
The protein material eluted from an anion exchange step during the industrial scale purification of proteins is of high added value, due to the high purity attained. Therefore, recovery is an essential parameter for productivity assessment during the technological process and a control parameter during the purification of the rHBsAg at industrial scale. In this regard, the lower control limit for recovery of rHBsAg after TMAE anion exchange in the established industrial process is set at 65 %, according to SOP 2.02.630.91 [21]. Thus, it was first determined rHBsAg recovery by using Cellufine MAX Q-hv and Capto Q chromatographic matrices.
Recovery values for these two resins are shown in figure 1, corresponding to three chromatographic runs each, together with the value of rHBsAg recovery obtained by using the TMAE resin during validation as performed in 2015 (87.48 ± 0.6 %). Mean values of recovered samples were: 82.07 ± 1.76 % for Cellufine MAX Q-hv and 87.16 ± 1.34 % Capto Q. As can be observed, these values are far above the standard lower limit set for the industrial process (65 %), even for TMAE (Figure 1).
The multiple-range test showed statistically significant differences between the recoveries of Cellufine MAX Q-hv and Capto Q resins, the latter having the highest mean recovery of rHBsAg. There were also no statistically significant differences between the recovery obtained for Capto Q and that of the TMAE resin (industrial process). Hence, attending only to this parameter, the Capto Q resin would be the best substitute of the TMAE resin. However, the Cellufine MAX Q-hv resin also complies with the required recovery value as established for the indus-trial process.
Adsorption kinetics
The adsorption kinetics describes the adsorption rate of the adsorbate in the adsorbent and determines the time at which equilibrium is reached. For this, it was represented the ratio between the final and initial concentrations (C/Co) of the rHBsAg in the adsorbate against the adsorption time (t). Samples were run in duplicate for each resin [22].
As shown in figure 2, the adsorption kinetics of rHBsAg for both resins followed the same profile. For Cellufine MAX Q-hv, C/Co ratio slowly decreased from 1 down to 0.15, lasting nearly 300 min to reach the equilibrium state of the resin and remaining constant afterwards at 0.15. It was estimated that a contact time between the rHBsAg and the gel around of about 5 h are required to saturate the chromatographic resin. In the case of Capto Q resin, the C/Co ratio also declined starting at 1 until reaching the equilibrium state at 0.03, but in about 200 min, faster than for Cellufine MAX Q-hv. Thus, it takes about 4 h to saturate the resin with the rHBsAg resin. Consequently, attending to the shorter contact time required to saturate the resin, the Capto Q resin is more advantageous than Cellufine MAX Q-hv.
Adsorption thermodynamics analysis by adsorption isotherms
Isotherms were performed with samples of pure adsorbate at a high concentration. Langmuir and Freundlich’s models were established to describe the amount of solute adsorbed by adsorbent (q) and solute concentration in solution (C) [23-25]. Both types of models are shown in Figure 3. These models were adjusted using nonlinear regression models by the Marquardt estimation method.
Capto Q resin
Langmuir and Freundlich’s models showed R2 values of 90.23 and 97.64 %, and standard deviation of the residues of 0.0848 and 0.04173, respectively (Figure 3 A and B). Although Freundlich’s model had a better fit, it has as limitation that it does not predict saturation of the adsorption surface and the equation that describes its performance is mainly empirical. On the other hand, Langmuir’s equation allows determining the maximum adsorption capacity (q* max) and the rHBsAg association constant (KA) for the resin. This model is supported by an equation with theoretical basis, also including several considerations for its application, such as: monolayer and reversible adsorption, homogeneous adsorption surface and the lack of interaction between the adsorbed molecules. It is reported in literature that Langmuir’s isotherm is an excellent approximation to the adsorption equilibrium of a single component and it is recommended its application when there is a strong specific interaction between the solute and the adsorbent to be studied. In this regard, the ion exchange and affinity adsorption processes can generally be described by the Langmuir’s isotherm [25]. The adjusted model equation resulted in:
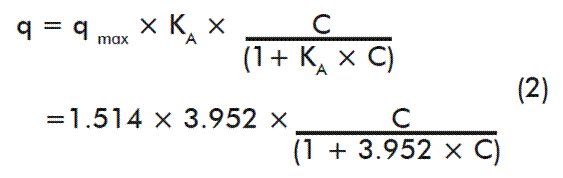
There were found qmax and KA values of 1.514 mg HBsAg/mL resin and 3.952mL/mg, respectively, by comparing the model with the equation.
Cellufine MAX Q-hv Resin
Figures 3C and 3D show Langmuir and Freundlich’s adjusted models, respectively, to describe the interaction between q and C. These models were fitted using nonlinear regression models. Langmuir and Freundlich’s models show R2 values of 95.11 and 98.08 %, respectively, and standard deviation of the residues of 0.0609 and 0.0382. As for the Capto Q resin, the Freundlich’s model had a better fit. Nevertheless, Langmuir’s model was selected to determine qmax and KA, since the Freundlich’s model is unable to predict the saturation of the adsorption surface. The equation of the adjusted model resulted in:
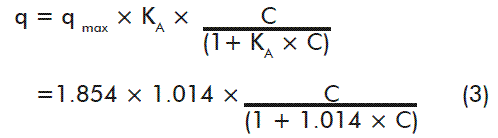
From this equation qmax and KA were obtained, with values of 1.854 mg HBsAg/mL resin and 1.014 mL/mg, respectively.
The Capto Q resin had an binding association constant 3.9 times higher than the Cellufine MAX Q-hv resin. The highest KA value for the Capto Q resin matched the shorter time required to reach equilibrium in kinetic studies, since KA is the ratio between the adsorption and desorption reactions rate constants. Additionally, the maximum adsorption capacities showed similar values for both chromatographic matrices.
From the theoretical physico-chemical point of view, a determination of adsorption isotherm requires its realization in a thermodynamic equilibrium state, which is more rigorously guaranteed in batch or stationary conditions, once the adsorption kinetics has been studied, to establish the time necessary to achieve the equilibrium state. Nevertheless, stationary do not fully resemble all the operating conditions in which the purification process is performed, since they provide a large specific surface and, therefore, the maximum possibility of adsorption. Besides, it can be considered that the flow is null, a condition that unmet in a column. Yet, Guiochon et al. showed that when the experiments are performed correctly, the isotherms obtained by stationary and frontal methods yield comparable results [25].
The good fit of the Langmuir model allows considering that a homogeneous monolayer adsorption occurs. On the other hand, the KA value obtained for both resins meet the criterion reported by Kumar et al. [26], according to which the dissociation constant (the inverse of the association constant) for ion exchange resins must have values in the range 10-8-10-4 mol/L. Given the molar mass reported for rHBsAg [27, 28], these values corresponded to 1.04 × 10-7 mol/L and 4.1 × 10-7 mol/L for Capto Q and Cellufine MAX Q-hv, respectively.
Determination of the dynamic capacity of resins
The dynamic adsorption capacity of the Cellufine MAX Q-hv and Capto Q chromatographic resins was determined by obtaining the breakthrough curve of each matrix. This experiment was aimed to determine how the concentration of solute varies over time at the chromatography outlet. The residence time of the adsorbate in the adsorbent was set to 0.7 h, equal to that used in the industrial process. The dynamic capacity or useful capacity of the chromatographic bed (q) was determined assuming as 0.05 the C/Co criterion at the bed outlet (breakthrough point determination) [11, 29]. The breakthrough curves obtained for the Cellufine MAX Q-hv and Capto Q chromatographic resins are similar to each other (Figure 4). The mass transfer zone for both resins is relatively narrow.
The dynamic properties obtained from the breakthrough curves for both resins are shown in Table 2. The adsorption capacity of Cellufine MAX Q-hv resin (1.5 mg rHBsAg/mL of resin) was higher than that obtained for Capto Q resin (0.9 mg rHBsAg/mL of resin). These values are approximately 100 times lower than those reported by manufacturers for BSA model ratings. Even though the matrix exclusion limits are not available, when comparing the molar masses of rHBsAg (2400 kDa, theoretical value calculated for the original native viral particle of HBV consists of 100 monomers) [30] and BSA (66.38 kDa), shows that the former is 36 times greater than the second. Therefore, this difference in the dynamic capacity can be explained by considering that the rHBsAg molecule purified as a virus-like particle does not diffuse into the pore, thereby, the surface effective for the adsorption is significantly reduced. Cellufine MAX Q-hv breakthrough times are higher than those obtained for the Capto Q matrix. The bed fraction used (UBF) for Cellufine MAX Q-hv and Capto Q resins showed values of 59.04 and 53.7 %, respectively (Table 2).
Overall, under the operating conditions described for this experiment, more than 40 % of the chromatographic bed for both resins is not being used efficiently. This indicates it would be possible to find new operating conditions that increase efficiency in the use of the column.
According to the results of the table, the greater dynamic capacity achieved for the Cellufine MAX Q-hv resin would lead to the use of a smaller volume of resin to process the same amount of rHBsAg, at a higher bed usage. These aspects would be advantage for this resin, if the useful life times of both were similar. This point required further investigation.
CONCLUSIONS
The chromatographic resins studied showed rHBsAg recoveries above the lower limit of industrial process control. There were statistically significant differences between the recovery values of both commercial Cellufine MAX Q-hv and Capto Q resins, with the Capto Q matrix showing the highest protein recovery (87.16 %). Their binding association constants were 1.014 for Cellufine MAX Q-hv and 3.952 mL/mg for Capto Q, with similar maximum adsorption capacities, 1.85 and 1.51 mg HBsAg/mL of resin, respectively. Conversely, the Cellufine MAX resin Q-hv displayed the highest dynamic adsorption capacity (1.5 mg rHBsAg/mL of resin) as compared to Capto Q (0.9 mg rHBsAg/mL of resin). Overall, these results support the replacement of the TMAE matrix by one of these two new anion exchange matrices for the purification of the rHBsAg at industrial scale. The choice will depend on the design and performance of the implemented purification process.
REFERENCES
1. Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384(9959):2053-63.
2. Organización Mundial de la Salud. Nota descriptiva #204, Hepatitis B. OMS, Julio 2015.
3. Flaquet PP. Aportes a la evaluación de la vacuna recombinante cubana contra la hepatitis B. CubaEditorial universitaria; 2009.
4. Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4(3):74-80.
5. Aguilar A, González CA, Cinza Z, Cabrera J, Veliz G, Moreno SR, et al. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int J Infect Dis. 2007;11:394-401.
6. Al-Mahtab M, Akbar SM, Aguilar JC, Uddin MH, Khan MS, Rahman S. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatol Int 2013;7:981-9.
7. Martínez E, Expósito N, Muzio V, Vega JL, Herrera-Martínez L. Desarrollo de vacunas combinadas. In: Rojas Ochoa F, editor. Vacunas. Cuba 1959-2008. La Habana: Editorial Ciencias Médicas; 2011. p. 185-90.
8. Doran PM. Bioprocess engineering principles. Amsterdam: Elsevier Science & Technology Books; 1995.
9. Scopes RK. Protein purification: principles and practice. Dordrecht: Springer Science & Business Media; 2013.
10. Gu T. Mathematical modeling and scale-up of liquid chromatography: With application examples. Springer; 2015.
11. Hagel L, Jagschies G, Sofer G. Handbook of process chromatography. The Netherlands: Academic Press; 2008.
12. Asenjo JA, Andrews BA. Protein purification using chromatography: selection of type, modelling and optimization of operating conditions. J Mol Recognit. 2009;22(2):65-76.
13. Fekete S, Beck A, Veuthey JL, Guillarme D. Ion-exchange chromatography for the characterization of biopharmaceuticals. J Pharm Biomed Anal. 2015;113:43-55.
14. Foo K, Hameed B. Insights into the modeling of adsorption isotherm systems. Chem Eng J. 2010;156(1):2-10.
15. Hagel L, Jagschies G, Sofer G. Handbook of process chromatography: development, manufacturing, validation and economics. London: Academic Press; 2008.
16. Bergander T, Nilsson-Valimaa K, Oberg K, Lacki KM. High-throughput process development: determination of dynamic binding capacity using microtiter filter plates filled with chromatography resin. Biotechnol Prog. 2008;24(3):632-9.
17. Madruga YA Cromatografía de Intercambio Iónico (1). PPO 2.02.610.91 CIGB. 2015.
18. Madruga YA. Cromatografia de Afinidad con Anticuerpos Monoclonales anti- HBsAg. PPO 2.02.615.91. CIGB. 2016.
19. Madruga YA. Determinación de absorbancia como control de proceso de la etapa de purificación del r-HBsAg. PPO 2.02.622.91 CIGB; 2008.
20. Smith I. Chromatography. The Netherlands Elsevier; 2013.
21. Madruga YA Cromatografía de intercambio iónico positivo. PPO 2.02.630.91 CIGB; 2008.
22. Rathore A, Krishnan R, Tozer S, Smiley D Rausch S, Seely J. Scaling down of biopharmaceutical unit operations: Part 2: Chromatography and filtration. Biopharm International. 2005;18(4):58-64.
23. Nugent P, Belmabkhout Y, Burd SD, Cairns AJ, Luebke R, Forrest K, et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature. 2013;495(7439):80-4.
24. Tang H, Zhou W, Zhang L. Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater. 2012;209-210:218-25.
25. Guiochon G, Felinger A, Shirazi D, Katti A. Fundamentals of preparatives and nonlinear chromatography. The Netherlands: Elsevier Academic Press; 2006.
26. Kumar P, Lau PW, Kale S, Johnson S, Pareek V, Utikar R, et al. Kafirin adsorption on ion-exchange resins: isotherm and kinetic studies. J Chromatogr A. 2014;1356:105-16.
27. Domínguez P. Evaluación de las resinas cromatográficas Cellufine MAX Q-h, MAX Q-hv, Capto Q ImpRes y Capto Q en la purificación del r-HBsAg. Tesis de Maestría en Ingeniería de los procesos biotecnológicos: Universidad Tecnológiga de La Habana; 2016.
28. Yan L, Jinxiu B,Weibin Z, Yongdong H, Lijing S, An-Ping Z, et.al. Characterization of the large size aggregation of Hepatitis B surface antigen (HBsAg) formed in ultrafilration process. Process Biochemestry. 2007;42:315-9.
29. Ghosh R. Principles of bioseparations engineering. Singapore: World Scientific; 2006.
30. Agraz A, Quinones Y, Exposito N, Brena F, Madruga J, Penton E, et al. Adsorption-desorption of recombinant hepatitis B surface antigen (r-HBsAg) from P. pastoris on a diatomaceous earth matrix: Optimization of parameters for purification. Biotechnol Bioeng. 1993;42(10):1238-44.
Received in August, 2016.
Accepted in April, 2017.
Pedro Domínguez Aldás. Centro de Ingeniería Genética y Biotecnología, Ave 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba. E-mail: pedro.dominguez@cigb.edu.cu.














