Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.35 no.1 La Habana ene.-mar. 2018
RESEARCH
Stability, safety and protective immunity of Gavac® vaccine subjected to heat stress
Estabilidad, seguridad e inmunidad protectora de la vacuna Gavac® sometida a estrés térmico
Milagros Vargas-Hernández1, Elaine Santana-Rodríguez1, Carlos Montero-Espinosa1, Yusmel Sordo-Puga1, Andy Acosta-Hernández2, Yoandy Fuentes-Rodríguez1, Danny Pérez-Pérez1, Aymé Oliva-Cárdenas1, Ernesto González-Ramos1, Carlos A Duarte4, Alain Moreira-Rubio3, Ileana Sánchez-Ortiz3, Marilin Domingo-Puentes3, Licette Leon-Barreras3, William Pena3, Marisela Suárez-Pedroso1
1 Departamento de Biotecnología Animal, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, La Habana, CP 11600, Cuba.
2 CPA Héroes de Yaguajay, Camagüey, Cuba.
3 Center for Genetic Engineering and Biotechnology, Camagüey, Cuba.
4 Departamento de Farmacéuticos, Dirección de Investigaciones Biomédicas, CIGB, La Habana, Cuba.
ABSTRACT
Gavac® is a commercial vaccine for the control of cattle tick (Rhipicephalus (Boophilus) microplus), containing the recombinant Bm86 protein antigen. The vaccine is supplied in multi-dose vials and the recommended application schedule includes two initial doses on days 0 and 28, followed by a boosting dose every 6 months. As any other vaccine, it requires cold chain conditions for storage and transportation, since heat stress conditions could affect vaccine efficacy. However, remaining vaccine doses once the vials are punctured, particularly in multidose vials, could generate a substantial loss of product due to the risk of vaccine instability. Therefore, this work was aimed to assess the safety and efficacy of the vaccine subjected to thermal stress in punctured vials to collect relevant information regarding the robustness of this immunogen under field conditions. It was found that pre-incubation of punctured vials at 37 °C up to 14 days neither increased the number of adverse reactions in experimental animals not the physicochemical properties of the emulsion. No statistical differences in the anti-Bm86 antibody titers were found after 7 days of incubation at 37 °C. Moreover, after 15 days of heat stress, Gavac® was still capable of eliciting protective antibody titers, with the ability to affect the reproductive parameters of ticks. These results demonstrate the stability, safety and efficacy of Gavac® under these conditions and reinforce the robustness of the vaccine under field applications.
Keywords: cattle tick, vaccination efficacy, immunogen Gavac®, protein Bm86, Rhipicephalus (Boophilus) microplus.
RESUMEN
Gavac® es una vacuna comercial para el control de la garrapata bovina (Rhipicephalus (Boophilus) microplus) que emplea la proteína Bm86 como antígeno recombinante. Se fabrica en viales multidosis y se administra en esquemas de dos dosis en los días 0 y 28, seguida de una dosis de refuerzo cada seis meses. Como otras vacunas, Gavac® requiere de condiciones de cadena de frío durante su almacenamiento y transportación, pues las condiciones de estrés térmico pueden afectar su eficacia. Sin embargo, en los viales multidosis, las dosis de vacuna remanentes tras el primer uso pueden conllevar a una pérdida sustancial de producto por el riesgo de inestabilidad. En este trabajo se evaluó la seguridad y eficacia de la vacuna sometida a estrés térmico en viales con un uso,para simular las condiciones de campo. La vacuna remanente en los viales tras una punción e incubados a 37 °C por 14 días no incrementó el número de reacciones adversas en el ganado bovino vacunado, ni alteró las propiedades fisicoquímicas de la emulsion. No se observaron diferencias estadísticamente significativas en los títulos de anticuerpos anti-Bm86 después de siete días de incubación a 37 °C. Además, tras 15 días de incubación a 37 °C, la vacuna mantuvo su capacidad para inducir respuesta protectora de anticuerpos, suficiente para afectar los parámetros reproductivos de las garrapatas. Estos resultados demostraron la estabilidad, seguridad, eficacia y robustez de Gavac® bajo condiciones de campo.
Palabras clave: garrapata bovina, eficacia de vacunación, immunógeno Gavac®, proteína Bm86, Rhipicephalus (Boophilus) microplus.
INTRODUCTION
Rhipicephalus (Boophilus) microplus is the most important cattle tick in tropical and subtropical areas [1]. This species has great veterinary importance since it is able to transmit viruses, bacteria, protozoa and rickettsiae [2]. In addition, ticks are responsible for severe economic losses in milk production, decreased weight gain and damage to skin of the animals by biting [1-4].
During the last decade, some strategies to control ectoparasites affecting animal production have been implemented, most of them involving chemical treatments. But, unfortunately, they have been unable to prevent and control the steady development of resistance to the chemicals in use today, despite their high effectiveness and practical use and with considerable environmental impact [5].
For these reasons, strategies for tick control based on integrated pest management have been proposed as the current alternative to delay the phenomenon of resistance to chemicals and to lower the environmental burden. The concept of control is based on integrated management systems alternating chemical treatments and control with acaricides scheduled according to the infestation index [6], or combined with biological and mechanical control, immunological and genetic control, among others. The main goal is to reach the greatest economic benefit for the producer at affordable costs and in an environmental-friendly scenario [7].
Inserted in the immune control is Gavac®, a vaccine based on the Bm86 antigen, isolated from gut of the tick Rhipicephalus (Boophilus) microplus [8-10] and expressed in the methylotrophic yeast Pichia pastoris. This vaccine, which has been useful to control tick infestations [11], is linked to a program of integrated tick control. Among other major measures, the program requires a change in the methodology of implementation of the acaricides baths [12]. The vaccine is applied following an immunization schedule consisting of two initial doses at weeks 0 and 4 and booster doses every six months [13]. This scheme has proved effective in inducing high antibody titers in cattle regardless of race, sex or productive category [14-17].
Gavac® is distributed in multidose vials, which are practical for field application; however, on many occasions, there are potentially useful doses left over in the vials. However, using these remaining doses under field conditions is not recommended, since it is assumed that their safety and immunogenicity could be affected after handling during endpoint applications [18]. Therefore, in this work, the stability, safety and immunogenicity of Gavac® vaccine doses remaining in punctured vials exposed to thermal stress were studied.
MATERIALS AND METHODS
Heat stress experiment
Bottles from a production lot of Gavac® (M22131-1) were used. Each vial contains 15 doses of 2 mL (100 µg of Bm86 protein each dose) for a total volume of 30 mL. The bottles were punctured with a needle for a first extraction and kept at different temperatures according to the following experimental design: groups I and II were preserved at 4 ºC for 14 days; group I was used as the positive control of the experiment, therefore the bottles of this group were preserved intact. Vaccines from groups III, IV and V were kept at 37 ºC for 3, 7 and 14 days, respectively. A sentinel group of six non-vaccinated animals in the same farm were used as negative controls in this experiment.
Determination of vaccine organoleptic properties
The effects of heat stress on the organoleptic characteristics of the vaccine bottles were evaluated by visual observation. Three vaccine vials for each temperature group were used. The bottles were opened. The content was poured in 15 mL tubes and allowed to settle in upright position for 10 min, and the contents of the tubes visually inspected for color and appearance. The emulsion must be bright white with a homogeneous appearance to past the test, according to the manufacturer's instructions [19].
Thermal stability
The test was designed to determine the stability of the emulsion after exposure to elevated temperatures. A sample of 10 mL of the emulsion was poured into 15 mL centrifuge tubes. The height of the emulsion (Ho) in the tube was measured. The tubes were kept for 15 days in an incubator at a temperature of 35 ± 2 ºC, and the height of the emulsion was measured again after that time (Hu). The Hu/Ho ratio was then calculated. This ratio must be equal to or higher than 0.9 to comply with the quality standards established for the vaccine [19].
Sterility test
Volumes of 2 mL of Gavac® were extracted from a vial previously punctured and further incubated either at 4 °C or 37 °C, as described before, and afterwards, they were added to 200 mL of dispersant solution (Peptone + Tween 80). After homogenization of the mixture, a 20-mL sample was taken and added to flasks with different culture media (Tryptone Soy Broth for fungi and Thioglycolate for bacteria). Other flasks with the same media inoculated with 103 c.f.u./mL of the respective microorganisms were used as positive controls (Staphylococcus aureus, Pseudomonas aureginosa and Clostridium sporogenes for bacteria and Bacillus subtilis, Candida albicans, Aspergillus niger for fungi). Flasks were then incubated for 14 days at 30 to 35 ºC for bacteria and 20 to 25 ºC for fungi. Negative controls treated with each medium were included. Flasks were observed on days 3, 5, 7, 9, 11 and 14 for the presence of turbidity, biofilms, lumps or any other form of microbial growth.
Drop size distribution
Samples of 20 µL of the vaccines subjected to each treatment were diluted in 980 ?L of 10 % Montanide adjuvant solution (Seppic, France). The mixture was stirred gently to preserve the original drop sizes. The emulsion was then observed microscopically and a total of 100 drops were measured. To comply with the test, 80 % of the drops in the sample must have a diameter equal to or lower than 5 µm [19].
Immunization of animals with Gavac® under field conditions
Thirty bovines (crossbred Siboney) over 4 months of age, with a body weight higher than 100 kg and serologically negative for Bm86 were used. The animals were identified by ear-clips and randomly assigned to one of the 5 treatment groups specified above. Six animals were included per group. Vaccines were applied by deep intramuscular injection of 2 mL (100 µg) in the neck muscles with 16 gauge needles on days 0 and 28.
Collection of sera and measurement of anti- Bm86 antibodies titers by ELISA
Blood samples were taken on days 0, 28 and 56 of the study; the sera were extracted and kept at -20 °C until used. The anti-Bm86 antibody titers were determined by ELISA as previously described [20]. Briefly, ELISA microplates (Nunc, Polysorp) were coated with Bm86 protein at 2 µg/mL and incubated overnight at 4 ºC. Subsequently, plates were washed with PBS-Tween 20 (0.05 %) and blocked with 1 % skimmed milk for 1 h at 37 ºC. Sera were next diluted 1:500 in PBS-Tween 20 (0.05 %) and 100 µL added to each well and further incubated for 1 h at 37 ºC. Next, a rabbit anti-bovine IgG antibody conjugated to horseradish peroxidase was added for 30 min at 37 ºC. Finally, the reaction was developed with 0.5 mg/ mL Ortho-phenylenediamine and 0.015 % H2O2 and the reaction stopped with H2SO4 (1 %). The optical density (OD) was measured at 492 nm in an ELISA plate reader (Sunrise-Austria). Individual titers were determined by interpolating OD units using a standard curve of serial 1:2 dilutions of a positive control serum diluted 1:640 (AU).
Safety evaluation
The onset of any event that could be associated to the administration of the immunogen was monitored through clinical observation and palpation and recorded. These included clinical signs and prostration, inflammatory reactions at the injection site, appreciable changes in respiratory rate or any other event [21].
Effect of the immunization with Gavac® on the reproductive capacity of ticks
The teleogines naturally occurring on the animals were collected at 56 days of the experiment. The following parameters were calculated: weight of engorged ticks, weight of the eggs, reproductive efficiency index and percentage of hatching. Ticks were placed at 28 ºC, 80 % relative humidity and a 12-h light:darkness photoperiod for egg laying and hatching. Reproductive efficiency index was determined as the weight of the egg mass (g)/weight of ticks (g) × 100. Hatching was determined by counting the eggs and larvae [22]. The efficacy of the vaccine was estimated by the following formulas [23]:
- Effects of immunization in the weight of ticks:
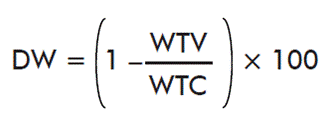
where WTV is the mean weight of ticks on the immunized cattle and WTC is the mean weight of ticks on control cattle.
- Impact on oviposition:
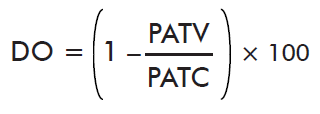
where PATV is the mean weight of eggs laid by ticks on the immunized cattle and PACT is the mean weight of eggs laid by ticks on control cattle.
- Effect on reproductive efficiency index:
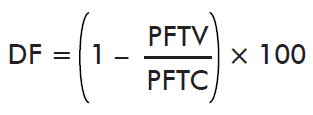
where PFTV is the mean efficiency index of the immunized group and PFTC is the mean efficiency index of the control group. Reproductive efficiency index is determined as the total weight of eggs mass (g) over the total weight of ticks (g) (%).
- Effect on Hatching:
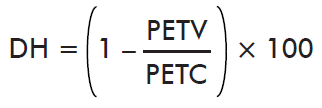
where PETV is the mean hatching by ticks collected on the immunized cattle and PETC is the mean hatching by ticks collected on control cattle.
Statistical analysis
Analysis of variance (ANOVA single factor) was used to compare the anti-Bm86 antibody titers and the reproductive parameters of ticks among groups. Turkey’s test was applied for post hoc comparisons (α = 0.05). Statistical processing was performed with the GraphPad Prism program version 6.0 (GraphPad software; La Jolla, USA).
RESULTS
Effect of heat stress on the organoleptic properties of Gavac®
No physical changes were detected in Gavac® emulsions when the open bottles were kept at 4 ºC or 37 ºC up to 14 days. No alterations were found in the appearance, consistency, color, smell, aggregates formation, sedimentation of the formulation. No separation in water/oil phases was observed. The Hu/Ho ratio mean was higher than 0.9 for all groups (Table 1).
Drop size distribution
Gavac® bottles kept at different temperatures (4 ºC or 37 ºC) for 3, 7 and 14 days maintained the same droplet size distribution. No emulsion breakage occurred and there was no formation of larger droplets since the 100 % of the drops were below 5 ?m in diameter in all the samples analyzed (Table 1).
Sterility test
No microorganism growth was observed in any of the vaccine bottles included in the study during the 14 days of the experiment.
Effect of heat stress on the immunogenicity of Gavac® in cattle
The influence of heat stress on the immunogenicity of Gavac® was evaluated by measuring the anti-Bm86 antibodies titers elicited in the serum of the immunized animals. A specific antibody response was detected in all experimental groups 28 days after the first injection. No statistically significant differences were found among groups, with mean titers ranging from 1: 4759 to 1: 17 467 (Figure 1). The anti-Bm86 titers increased after the second dose in all groups and statistically significant differences were found among them (p < 0.05). In particular, the mean antibody titer for group V (1:5,435) was more than five times lower than in the control group I (1:28 353) (p < 0.05).The differences among the rest of the groups were not statistically significant.
Effect of vaccination on the reproductive parameters of ticks
A significant affectation in the reproductive parameters of ticks (weight of ticks, weight of eggs, reproductive efficiency index and hatching percentage) as compared to control animals was documented at day 56 of the experiment, regardless of the temperatures and incubation times tested (Table 2). The observed reductions in these parameters were similar for all treatment groups.
The weights of engorged ticks and eggs were significantly lower in all groups as compared to non-immunized controls (p < 0.0001). No differences among vaccinated groups (p > 0.05) were detected. The reproductive efficiency index and hatching percentages were also similar among all vaccinated groups (Figure 2) and differed significantly (p < 0.01) from non-vaccinated controls.
DISCUSSION
Vaccines are capable of inducing a protective and durable immune response in animals, but often some mild side effects such as local reactions, fever and general symptoms are observed as a consequence of the associated immune inflammatory response [24-26]. Vaccines can be very susceptible to environmental conditions. In particular, temperature changes significantly affect the integrity of this type of product during storage, transportation and handling. This issue is particularly sensitive for live-attenuated vaccines [27, 28].
The recombinant subunit vaccines could mitigate these problems. Generally, the purified recombinant proteins are very stable when subjected to temperature changes for short periods of time. Additionally, oil based adjuvants tend to increase the stability of protein antigens [29, 30].
Among all the methods available today for the control of cattle tick, vaccination with recombinant Bm86-based vaccines has been the most effective and environmentally friendly [9-11, 15, 31-36]. The simultaneous immunization of large groups of animals in a rural setting allows the use of multi-dose vials. It is therefore important to study the stability of the vaccine doses remaining in punctured vials under increased temperature conditions. Based on these results, it is proposed that remaining vaccine doses in multidose vials after a vaccination event could be preserved for a single temperature cycle at 4 ºC for up to 7 days, and administered immediately after in a new vaccination event. It is not recommended by no means to subject the vaccine vial to subsequent temperature cycles, since protein stability cannot be guaranteed at all. It is of outmost importance that all vaccine handling should be carried out following the standard good handling practices, to guarantee the consistency and efficacy of results. Moreover, it is strongly advised to follow the manufacturer's recommendations for the safe and efficacious use of Gavac® vaccine, and the use proposed in this work could be only limited to vaccine losses representing half the doses contained in the vial.
The stability of vaccines has been associated with the resistance to light-mediated physical degradation, temperature changes during storage and transportation, and the time elapsed from manufacture. Maintaining drop size between the established parameters preserves the emulsion stability [37]. Small droplet sizes allow a more efficient diffusion of the antigen when injected in the animal, reaching more quickly the systemic lymphatic tissues and, thus, triggering the immune response of the animal more effectively [37].
In this study, the stability of Gavac® after 14 days of heat stress at 37 ºC in open multi-dose vials was assessed. First of all, no separation of the aqueous and oil phases was observed after this incubation period. Second, the droplet size distribution remained within the criteria of quality and stability of the emulsion. Third, no microorganism growth or changes in the organoleptic properties of the immunogen were detected. All these results indicated that the physicochemical properties of the emulsion were preserved.
Next, the immunogenicity of the vaccine preparations subjected to heat stress was assessed in cattle through a biphasic immunization schedule (immunization on weeks 0 and 4). The results of the antibody titers after two inoculations demonstrated that the vaccine preserved near full potency after 7 days at 37 ºC. A reduction in the antibody titers was observed after 14 days of incubation a 37 ºC. This outcome was inferred though remaining to be demonstrated, since it is well known that increased temperatures favored both, protease mediated and independent degradation of protein antigens [38]. Although no changes were observed in the emulsion in vials incubated at 37 ºC for 14 days, this may affect the structure and the functional properties of the protein, particularly its capacity to elicit antibody responses against conformational epitopes. However, taking into account that antibody titers equal or above 1:640 are considered protective [13, 15], even animals from Group V (37 ºC for 14 days) can be regarded as protected from cattle tick infestation.
The kinetics of the antibody responses and the titers observed for the rest of the groups were quite similar to those reported in previous studies with this immunogen [15, 23, 35, 39-43]. All animals showed a detectable antibody response right after prime immunization and developed an anamnestic response characterized by increased antibody levels, similar to those previously described [13, 15, 23, 31, 39, 41-44].
No local or systemic adverse events were documented in immunized animals, despite the thermal stress treatments applied to the product. In this sense, results were similar to those described for the standard vaccine administration procedure, in which the Bm86 antigen has been demonstrated to be safe both, during the days around the time of immunization and after six months, when the administration site was examined by necropsy [23, 38].
Up to our knowledge, this is the first integral study of the sterility, stability and immunogenicity of a veterinary vaccine in open multi-dose vials subjected to thermal stress. Other studies on the thermal stability of veterinary vaccines have been conducted in closed vials. For instance, post-formulation modifications were seen in seven proteins tested during storage of 1 week at 37 °C in vaccines formulated in the oil adjuvant Montanide ISA 720, and these modifications had a negative impact in their immunogenicity [45]. In contrast, an experimental recombinant vaccine against classical swine fever, formulated in Montanide 888 was stable and conserved its immunogenicity after 1 week at 37 °C[46].
Overall, our studies provide useful information on the relative stability of veterinary vaccines during its manipulation under field conditions and at endpoint application. Noteworthy, this is informative and manageable, but does not substitute the conditions and recommendations made by manufacturers for optimal temperature conditions to obtain best results during vaccine transport, handling and administration to animals. In the specific case of Gavac®, manufacturers recommend that the product should be kept at 4 ºC during storage and transportation. From the results shown in this study, it can be concluded that vials in use can be kept at temperatures up to 37 ºC for one week without substantial loss of its immunogenicity and keeping its safety profile, under certified management procedures.
Despite the absence of recommendations or classifications by regulatory agencies for veterinary vaccines attending to their thermal susceptibility, equivalent criteria have been somewhat inherited from recommendations in place for human vaccines. Specifically, this has been the case for recommendations made by the American Animal Hospital Association, the accrediting body for companion animal hospitals in the United States and Canada. It recommends to follow standard procedures as established by manufacturers for multidose vials in terms of number of punctures and handling [47]. Moreover, this organization also recommends to endorse the Vaccine Storage and Handling Toolkit issued by the US Centers for Disease Control and Prevention (CDC) [48], where it is clearly stated that temperature fluctuation during transportation or storage can irreversibly affect the potency of vaccines.
Additionally, as the case for vaccines approved for human use, WHO has classified them in four groups according to their thermal stability: high stability (30 days at 37 °C); medium stability (14 days at 37 °C), moderate stability (7 days at 37 °C) and least stable (2 days at 37°C) [49]. According to this classification Gavac® can be classified as a medium stability vaccine, although more prolonged incubation times are yet to be studied. Nevertheless, as discussed before, our experiments were carried out with punctured vials; a situation that better resembles the real situation in the field for a veterinary vaccine, much more stressing than in the usual accelerated stability studies in the lab.
WHO policy for the use of multi-dose vaccines in humans recommends that multi-dose vials should be discarded at the end of the immunization session, or within six hours of opening, unless the vaccine meets the following criteria: 1) The vaccine is currently prequalified by WHO, 2) The vaccine is approved for use for up to 28 days after opening the vial; 3). The expiry date of the vaccine has not passed and 4) The vaccine vial has been and will continue to be stored at WHO or manufacturer recommended temperatures, and the vaccine has not been damaged by freezing [50]. These recommendations are supported by previous data on the use of multi-dose vaccines or other injectable drugs [51, 52].
Overall, the knowledge derived from this work is of practical importance for the implementation of vaccination campaigns in the field, mainly during endpoint application, further supporting a more efficient use by practical means of multi-dose vials as an effective cost-containment measure.
CONCLUSIONS
In summary, animals immunized with Gavac® vaccine doses remaining in multidose vials showed no adverse reactions and developed anti-Bm86 antibody titers equivalent to those immunized with control vaccine, when the vaccine was experimentally subjected to 37 ºC for a week. The antibody response retains its ability to experimentally reduce the weight and to impair the reproductive capacity of ticks. This allows to exceptionally store the doses remaining in multidose vials at 4 ºC for up to 7 days after heat stress during a vaccination campaign. In this way, all doses within multidose vials containing the Gavac® vaccine can be optimally used, when they are handled and managed under certified practices for cattle vaccination at endpoint applications, and when manufacturer's standard recommendations of discarding the remaining content could lead to considerable losses.
CONFLICTS OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
1. Casas E, Trigueros A, Chávez A, Tang J, Ruiz F. Tratamiento y control de Garrapata Boophilus microplus, a través de la combinación de fluazurón/ fipronilpouron, en bovinos de trópico. Pucallpa, Perú: Lima: Facultad de Medicina veterinaria, Universidad Nacional de San Marcos; 2009.
2. FAO. Tick and Tick Borne Diseases Control: A practical field manual. 2da Ed. Rome: FAO-UNDP; 1984.
3. Rojas M. Nosoparasitosis de los rumiantes peruanos. 2da Ed. Lima: Martegraf; 2004.
4. Urquhart GM, Armour J, Duncan JL, Dunn AM, Jenningsf W. Parasitología veterinaria. 2da Ed. Zaragoza: Acribia; 2001.
5. Nari A, Eddi CS. Control de la resistencia a los antiparasitarios a la luz de los conocimientos actuales. 2002 [cited 2018 Feb 18]. Available from: http://www.produccion-animal.com.ar
6. Suárez Pedroso M, Mellor LM, Valdez M, Souza RM, Camargo AJR, Vargas NC, et al. Control de las infestaciones de la garrapata Boophilus microplus en la ganadería Cubana y en regiones de latinoamérica con la aplicación del inmunógeno Gavac® dentro de un programa de lucha integral. 2007 [cited 2018 Feb 18]. Available from: http://www.corpoica.org.co/redectopar.asp
7. García A, Barral M. Métodos de control de las garrapatas. Ovis. 1999;65:1-9.
8. Gough JM, Kemp DH. Localization of a low abundance membrane protein (Bm86) on the gut cells of the cattle tick Boophilus microplus by immunogold labeling. J Parasitol. 1993;79(6):900-7.
9. Rand KN, Moore T, Sriskantha A, Spring K, Tellam R, Willadsen P, et al. Cloning and expression of a protective antigen from the cattle tick Boophilus microplus. Proc Natl Acad Sci U S A. 1989;86(24):9657-61.
10. Willadsen P, Riding GA, McKenna RV, Kemp DH, Tellam RL, Nielsen JN, et al. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J Immunol. 1989;143(4):1346-51.
11. Rodriguez M, Rubiera R, Penichet M, Montesinos R, Cremata J, Falcon V, et al. High level expression of the B. microplus Bm86 antigen in the yeast Pichia pastoris forming highly immunogenic particles for cattle. J Biotechnol. 1994;33(2):135-46.
12. Valle MR, Mendez L, Valdez M, Redondo M, Espinosa CM, Vargas M, et al. Integrated control of Boophilus microplus ticks in Cuba based on vaccination with the anti-tick vaccine Gavac. Exp Appl Acarol. 2004;34(3-4):375-82.
13. Vargas M, Montero C, Sanchez D, Perez D, Valdes M, Alfonso A, et al. Two initial vaccinations with the Bm86-based Gavacplus vaccine against Rhipicephalus (Boophilus) microplus induce similar reproductive suppression to three initial vaccinations under production conditions. BMC Vet Res. 2010;6:43.
14. Boue O, Redondo M, Montero C, Rodriguez M, de la Fuente J. Reproductive and safety assessment of vaccination with Gavac against the cattle tick (Boophilus microplus). Theriogenology. 1999;51(8):1547-54.
15. de la Fuente J, Rodriguez M, Redondo M, Montero C, Garcia-Garcia JC, Mendez L, et al. Field studies and cost-effectiveness analysis of vaccination with Gavac against the cattle tick Boophilus microplus. Vaccine. 1998;16(4):366-73.
16. Suarez M, Rubi J, Pérez D, Cordova V, Salazar Y, Vielma A, et al. High impact and effectiveness of Gavac™ vaccine in the national program for control of bovine ticks Rhipicephalus microplus in Venezuela. Livestock Sci. 2016;187:48-52.
17. Botello AR, Botello AL, Borroto CN, Suárez M, Pérez DA, Rodríguez YV, et al. Control de garrapatas Riphicephalus (Boophilus) microplus en bovinos con el inmunógeno Herber biogar. Rev Electrón Vet. 2011;12(5):1-10.
18. European Medicines Agency. Committee for Medicinal Products for Veterinary Use. Guideline on data requirements to support in-use stability claims for veterinary vaccines. EMA/CVMP/IWP/250147/2008. 2010 Mar 15 [cited 2018 Feb 18]. Available from: https://www.ema.europa.eu/documents/scientific-guideline/guideline-datarequirements-support-use-stability-claimsveterinary-vaccines_en.pdf.
19. Enríquez A, Canales M, Ramos E, Dandie H, Boué O, Soto A, et al. Production of a recombinant vaccine against Boophilus microplus. In: de la Fuente J, editor. Recombinant vaccines for the control of cattle tick. La Habana: Elfos Scientiae; 1995. p. 79-103.
20. Triguero A, Blanco R, Machado H, Rodriguez M, de La Fuente J. Development of enzyme linked immunosorbent assays to measure Bm86 antigen of Boophilus microplus (cattle tick) and to detect anti- Bm86 antibodies in serum samples. Biotechnol Tech. 1999;13(2):119-25.
21. European Medicines Agency, EMA. VICH Topic GL44. Step 7. Guideline on target animal safety for veterinary live and inactived vaccines. London: EMA; 2009.
22. Bennett GF. Oviposition of Boophilus microplus (Canestrini) (Acarida: Ixodidae). I. Influence of tick size on egg production. Acarologia. 1974;16(1):52-61.
23. Miller R, Estrada-Pena A, Almazan C, Allen A, Jory L, Yeater K, et al. Exploring the use of an anti-tick vaccine as a tool for the integrated eradication of the cattle fever tick, Rhipicephalus (Boophilus) annulatus. Vaccine. 2012;30(38):5682-7.
24. Baldrick P. Dose site reactions and related findings after vaccine administration in safety studies. J Appl Toxicol. 2016;36(8):980-90.
25. Lewis DJ, Lythgoe MP. Application of 'Systems Vaccinology' to evaluate inflammation and reactogenicity of adjuvanted preventative vaccines. J Immunol Res. 2015;2015:909406.
26. Stassijns J, Bollaerts K, Baay M, Verstraeten T. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among children. Vaccine. 2016;34(6):714-22.
27. Karp CL, Lans D, Esparza J, Edson EB, Owen KE, Wilson CB, et al. Evaluating the value proposition for improving vaccine thermostability to increase vaccine impact in low and middle-income countries. Vaccine. 2015;33(30):3471-9.
28. Purssell E. Reviewing the importance of the cold chain in the distribution of vaccines. Br J Community Nurs. 2015;20(10):481-6.
29. Singh M, O'Hagan DT. Recent advances in veterinary vaccine adjuvants. Int J Parasitol. 2003;33(5-6):469-78.
30. Spickler AR, Roth JA. Adjuvants in veterinary vaccines: modes of action and adverse effects. J Vet Intern Med. 2003;17(3):273-81.
31. Almazan C, Lagunes R, Villar M, Canales M, Rosario-Cruz R, Jongejan F, et al. Identification and characterization of Rhipicephalus (Boophilus) microplus candidate protective antigens for the control of cattle tick infestations. Parasitol Res. 2010;106(2):471-9.
32. Cunha RC, Andreotti R, Leite FP. Rhipicephalus (Boophilus) microplus: expression and characterization of Bm86-CG in Pichia pastoris. Rev Bras Parasitol Vet. 2011;20(2):103-10.
33. de la Fuente J, Almazan C, Canales M, Perez de la Lastra JM, Kocan KM, Willadsen P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim Health Res Rev. 2007;8(1):23-8.
34. de la Fuente J, Kocan KM. Advances in the identification and characterization of protective antigens for recombinant vaccines against tick infestations. Expert Rev Vaccines. 2003;2(4):583-93.
35. de Vos S, Zeinstra L, Taoufik O, Willadsen P, Jongejan F. Evidence for the utility of the Bm86 antigen from Boophilus microplus in vaccination against other tick species. Exp Appl Acarol. 2001;25(3):245-61.
36. Rodriguez-Valle M, Taoufik A, Valdes M, Montero C, Ibrahin H, Hassan SM, et al. Efficacy of Rhipicephalus (Boophilus) microplus Bm86 against Hyalomma dromedarii and Amblyomma cajennense tick infestations in camels and cattle. Vaccine. 2012;30(23):3453-8.
37. Gerdts V. Adjuvants for veterinary vaccines--types and modes of action. Berl Munch Tierarztl Wochenschr. 2015;128(11-12):456-63.
38. Kumar B, Murugan K, Ray DD, Ghosh S. Efficacy of rBm86 against Rhipicephalus (Boophilus) microplus (IVRI-I line) and Hyalomma anatolicum anatolicum (IVRI-II line) infestations on bovine calves. Parasitol Res. 2012;111(2):629-35.
39. Cobon G, Hungerford J, Woodrow M, Smith D, Willadsen P. Vaccination against Boophilus microplus. The Australian field experience. In: de la Fuente J, editor. Recombinant vaccines for the control of cattle tick. La Habana: Elfos Scientiae; 1995. p. 163-7.
40. de la Fuente J, Rodriguez M, Montero C, Redondo M, Garcia-Garcia JC, Mendez L, et al. Vaccination against ticks (Boophilus spp.): the experience with the Bm86-based vaccine Gavac. Genet Anal. Eng. 1999;15(3-5):143-8.
41. Fragoso H, Rad PH, Ortiz M, Rodriguez M, Redondo M, Herrera L, et al. Protection against Boophilus annulatus infestations in cattle vaccinated with the B. microplus Bm86-containing vaccine Gavac. Vaccine. 1998;16(20):1990-2.
42. Canales M, Almazan C, Naranjo V, Jongejan F, de la Fuente J. Vaccination with recombinant Boophilus annulatus Bm86 ortholog protein, Ba86, protects cattle against B. annulatus and B. microplus infestations. BMC Biotechnol. 2009;9:29.
43. Garcia-Garcia JC, Montero C, Redondo M, Vargas M, Canales M, Boue O, et al. Control of ticks resistant to immunization with Bm86 in cattle vaccinated with the recombinant antigen Bm95 isolated from the cattle tick, Boophilus microplus. Vaccine. 2000;18(21):2275-87.
44. Andreotti R. Performance of two Bm86 antigen vaccine formulation against tick using crossbreed bovines in stall test. Rev Brasileira Parasitol Vet. 2006;15(3):97-100.
45. Miles AP, McClellan HA, Rausch KM, Zhu D, Whitmore MD, Singh S, et al. Montanide® ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine. 2005;23(19):2530-9.
46. Barrera M, Sanchez O, Prieto Y, Castell S, Naranjo P, Rodriguez MP, et al. Thermal stress treatment does not affect the stability and protective capacity of goat milk derived E2-marker vaccine formulation against CSFV. Vet Immunol Immunopathol. 2009;127(3-4):325-31.
47. American Animal Hospital Association. AAHA canine vaccination guidelines. 2017 [cited 2018 Jan 18]. Available from: https://www.aaha.org/guidelines/canine_vaccination_guidelines/cdc_vaccine_storage_handling.aspx
48. U.S. Department of Health and Human Services, Center for Disease Control and Prevention. Vaccine storage & handling toolkit. Atlanta: Center for Disease Control and Prevention; 2018 [cited 2018 Feb 15]. Available from: https://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/storagehandling-toolkit.pdf
49. World Health Organization. Temperature sensitivity of vaccines. Geneva: Department of Immunization, Vaccines and Biologicals, World Health Organization; 2006.
50. World Health Organization. WHO policy statement: multi-dose vial policy (MDVP): handling of multi-dose vaccine vials after opening. Geneva: World Health Organization; 2014.
51. Sheth NK, Post GT, Wisniewski TR, Uttech BV. Multidose vials versus single-dose vials: a study in sterility and cost-effectiveness. J Clin Microbiol. 1983;17(2):377-9.
52. Christensen EA, Mordhorst CH, Jepsen OB. Assessment of risk of microbial contamination by use of multidose containers of injectable products. J Hosp Infect. 1992;20(4):301-4.
Received in November, 2017.
Accepted in March, 2018.
Milagros Vargas-Hernández. Departamento de Biotecnología Animal, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, La Habana, CP 11600, Cuba. E-mail: milagros.vargas@cigb.edu.cu.














