Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.35 no.3 La Habana jul.-set. 2018
TECHNIQUE
Effect of temperature on the analytical performance of the UMELISA® TIR NEONATAL assay for newborn screening of cystic fibrosis
Efecto de la temperatura sobre el desempeño analítico del ensayo UMELISA® TIR NEONATAL para la pesquisa neonatal de la fibrosis quística
Elisa M Castells-Martínez1, Amarilys Frómeta1, Pedro L Pérez1, Odalys Martín1, Lesley del Río1, Maryeris Espinosa1, Claudia Almira1, Zoe Núñez1, Ernesto C González-Reyes2
1 Neonatal Screening Laboratory, Immunoassay Center.25th Ave and 134 Street, Cubanacán, Playa, CP 6653, Havana, Cuba.
2 R&D Director. GK Pharmaceuticals CMO. Barrio Coto Norte Carr. #2 Km 45.7, Manatí, PR 00674, Puerto Rico.
ABSTRACT
UMELISA® TIR NEONATAL is a kit developed to quantify immunoreactive trypsin in newborns' dry blood spots on filter paper. Reagents were stored at -20, 23 and 37 °C for 2, 4, 6, 8, 14 and 20 days. The mean of the fluorescence values of the calibrators, the recovery percentage (%R) and the concentration of the assay control were calculated. The kit showed different behaviors at each temperature studied, obtaining %R higher than 80 %, when it was stored at -20 ºC. For 37 °C it was not possible to exceed 6 days of the test due to a decrease in %R. For 23 °C, at 14 days a decrease in %R was observed, but still permissible as described in the specifications of the assay. Temperature directly influences the analytical performance of the UMELISA® TIR Neonatal. The kit can be exposed to a temperature of -20 ± 2ºC for 20 days and at a temperature of 23 ± 2 ºC, for 14 days without affecting its functional characteristics but it cannot be stored at a temperature of 37 ± 2 ºC for more than 4 days, since its optimum quality parameters are affected. It was demonstrated that the quality parameters of the UMELISA® TIR NEONATAL are affected according to their storage conditions.
Keywords: UMELISA® TIR NEONATAL, temperature, recovery percentage, stability.
RESUMEN
El UMELISA® TIR NEONATAL es un diagnosticador desarrollado para cuantificar tripsina inmunorreactiva en manchas de sangre seca sobre papel de filtro de recién nacidos. Los reactivos se almacenaron a -20, 23 y 37 ºC durante 2, 4, 6, 8, 14 y 20 días. Se calculó la media de los valores de fluorescencia de los calibradores, el porcentaje de recuperación (%R) y la concentración del control del ensayo. El diagnosticador mostró comportamientos diferentes en cada temperatura estudiada, obteniéndose %R superiores al 80 %, para el almacenamiento a -20 ºC. Para 37 ºC no fue posible sobrepasar los 6 días de la prueba debido a una disminución en el %R. Para 23 ºC, a los 14 días se observó una disminución del %R, pero aún permisible según lo descrito en las especificaciones del ensayo. La temperatura influye directamente sobre el desempeño analítico del UMELISA® TIR NEONATAL. El estuche de reactivos puedes ser almacenado a una temperatura de -20 ± 2 ºC durante 20 días ya una temperatura de 23 ± 2 ºC, durante 14 días sin que se afecten sus características funcionales, pero no puede ser almacenado a una temperatura de 37 ± 2 ºC por más de 4 días ya que los parámetros óptimos de calidad se ven afectados. Se demostró que los parámetros de calidad del UMELISA® TIR NEONATAL, se afectan según sus condiciones de almacenamiento.
Palabras clave: UMELISA® TIR NEONATAL, temperatura, porciento de recuperación, estabilidad.
INTRODUCTION
Cystic fibrosis (CF) is one of the most common autosomal recessive disorders in the Caucasian populations, affecting approximately 1 in 3000 newborns [1-4]. Early identification of CF through newborn screening (NBS) is associated with improvements in the nutritional, respiratory, gastrointestinal and cognitive function, as well as in the overall survival of patients suffering from the disease [5-8].
In 1979, the development of a test to measure immuno-reactive trypsinogen (IRT) in dried blood spots made feasible universal NBS for CF. It also provided laboratories with a potential screening test to identify infants with CF in the first weeks of life, often before they presented clinical signs [9].
The UMELISA® TIR NEONATAL is a kit of reagents developed by the Immunoassay Center (CIE, acronym in spanish) for quantification of IRT in newborns' dry blood spots on filter paper, which is inserted in the platform of Ultra Micro Analytic techniques (SUMA, acronym in spanish) and ultramicroELISA tests (UMELISA®) developed in Cuba in the 1980's of the past century. These tests combine the high sensitivity of current microELISA assays, with the use of ultramicro volumes of samples and reagents [10].
Stored biopharmaceuticals products change as they age, but they are considered stable as long as their characteristics remain within the manufacturers' specifications [11]. The shelf life of each product, the number of days that a product remains stable under the recommended storage conditions, is estimated by performing stability tests. Temperature is the most common acceleration factor used for chemical, pharmaceutical and biological products, since its relationship with the degradation rate is characterized by the Arrhenius equation [11]. For more than 30 years, researchers have studied the relationship between temperature and marker stability for many of the congenital disorders that are detected in dried blood spots on filter paper, including IRT [12-16].
Therefore, taking into account the importance of knowing the influence of storage conditions on the stability of the components of this reagent kit, the aim of this work was to evaluate its quality parameters and functional characteristics when it was subjected to different storage temperatures.
MATERIALS AND METHODS
Chemicals
All reagents were of analytical grade and solutions were prepared in distilled water. Human trypsin was from Athens Research (USA). Tris, NaN3, NaCl, Na2HPO4, KCl, KH2PO4, diethanolamine, MgCl2, ZnCl2 and sucrose were purchased from Merck (Germany). Bovine serum albumin (BSA) and alkaline phosphatase were from Roche (Germany). HCl, Tween 20, glutaraldehyde, 4-methylumbelliferyl phosphate were from Sigma (USA).
Equipment and accessories
SUMA technology was used. The system is manufactured by the Immunoassay Center, Havana, Cuba, and includes instrumentation and reagents. Equipment comprises a fully computerized spectro-fluorimeter-photometer for the automatic reading, quantification, validation and interpretation of the results (PR-621) and a plate washer (MW-2001). The reagents are cased in kits in quantities enough for 288 ultra-microtests (10 μL volumes of samples and reagents) [10].
UMELISA® TIR NEONATAL reagents
The UMELISA® TIR NEONATAL is a heterogeneous 'sandwich type' immunoenzymatic assay for the determination of IRT in dried blood samples on filter paper. It uses as solid phase strips coated with anti-IRT monoclonal antibodies which were obtained in the CIE and are highly specific for IRT. Calibrators and control were prepared from washed hemolyzed erythrocytes and IRT-free plasma, adjusting the hematocrit to 55 % and spotted on Whatman 903 filter paper cards. A 1 mg/mL IRT solution (1 mg of IRT dissolved in 1 ml of 2 mM HCl) was used. The calibrators and control presented concentrations between 0-500 ng/mL and were prepared by gravimetric method. To prepare the conjugate, anti-IRT monoclonal antibodies were covalently bound to phosphatase alkaline by a reaction with glutaraldehyde in one step, according to the procedure described by Avrameas. The preparation of all these reagents has been previously described [17].
UMELISA® TIR NEONATAL technical procedure
For the measurement of IRT concentrations, 3 mm blood discs of standards, controls and samples were punched out of the filter paper and placed into each well of the elution microplates, followed by the addition of 70 μL of the diluted anti-IRT Mab-alkaline phosphatase conjugate in 0.05 mol/L Tris buffer, pH 8.0 containing 0.15 mol/L NaCl, 3 mmol/L NaN3, 2.9 mmol/L MgCl2, 2.5 mmol/L ZnCl2, 0.75 mol/L BSA and 1.1 mmol/L Tween 20. After the elution step in a humid chamber at room temperature for 16-18 h, 10 μL of eluate were transferred into the well of the reaction opaque polyestirene ultramicroplates coated with the specific anti-IRT Mabs. The immunological reaction occurred for 2 h room temperature in a humid chamber and then, the plates were washed six times with 0.37 mol/L Tris-HCl solution, pH 8.0 containing 3.76 mol/L NaCl, 1.1 mmol/L Tween 20 and 76.9 mmol/L NaN3. The fluorogenic reaction was performed by adding 10 μL of the substrate solution, pH 9.6 containing 5.07 mM 4-methylumbelliferil phosphate, 0.92 mol/L diethanolamine-HCl, 0.7 mmol/L MgCl2 and 7 mmol/L NaN3. The ultramicroplates remained at room temperature in a humid chamber for 30 min. Finally, the fluorescence was automatically measured in the fluorimeter-photometer reader. Automatic validation and interpretation of the results were done using a specific-assay software [17].
Protocol to evaluate the effect of temperature on the performance of UMELISA® TIR NEONATAL
Eighteen cases of the same batch were evaluated to determine the effect of storage conditions on the performance of the quality parameters and functional characteristics of the UMELISA® TIR NEONATAL. Six of these cases were stored at -20 ± 2 ºC; another six were stored at 23 ± 2 ºC and the remaining ones at 37 ± 2 ºC. The times selected for the study were: initial time, 2, 4, 6, 8, 14 and 20 days, taking as control the results of the stored kits of reagents at the temperature indicated in the external container (from 2 to 8 ºC). The assay was performed following the recommended user's instructions and it was evaluated according to the criteria established for the UMELISA® TIR NEONATAL.
For every time evaluated, the average of the fluorescence values and the recovery percentage (%R) for every calibrator was determined, using the following formula:
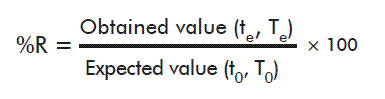
Where te and Te stand for time and temperature under evaluation, and t0 and T0 are the values of these parameters at the start of the experiment and 2-8 ºC, respectively.
IRT concentration values (ng/mL) of the assay's control for every time and temperature under study were quantified. The control calibration curve evaluated at the initial time was taken as reference. The relative accuracy percentage (%RA) was calculated by the following formula:

Data were processed using Microsoft Excel 2003 (Microsoft Corporation, USA).
RESULTS
It was previously demonstrated that the UMELISA® TIR NEONATAL is stable for twelve months stored at 2-8 ºC. Instead, there was the need to test its performance when the kits and reagents could be subjected to different temperature conditions during transportation and storage. Therefore, it was considered useful to evaluate the shelf stability of this reagent kit at different storage conditions. Three storage temperatures were studied: -20 ± 2 ºC, 23 ± 2 ºC and 37 ± 2 ºC. Results showing the behav-ior of the calibration curve for each case were as described below.
Assay performance stored at -20 ± 2 ºC
The %R of the fluorescence obtained for each calibrator was obtained for the UMELISA® TIR NEONATAL stored at -20 ± 2 ºC during the study (Table 1). This variable, defined as the error (%) between the observed or obtained value and the actual or expected value, was in the range 80-120 % for all the points of the curve during the 20 days of the study. This is the accepted range for this type of assay [18]. Hence, this demonstrates that UMELISA® TIR NEONATAL retain its quality parameters and functional properties when exposed to storage conditions of -20 ± 2 °C for up to 20 days.
Assay performance stored at 23 ± 2 ºC
Taking into consideration the reagent kits stored at room temperature (Table 2), it was shown that the %R for every calibrator were within the permissible range for this type of tests until day 14 at 23 ± 2 ºC. In the test carried out on day 20, values of %R below 80 % were obtained for all the points of the calibration curve, demonstrating the negative effect of these storage conditions on the adequate performance of the UMELISA® TIR NEONATAL.
Assay performance stored at 37 ± 2 ºC
Similar as for the test temperature 23 ± 2 ºC, the analytical performance of this reagent kit was affected at 37 ± 2 ºC during the assayed period. In fact, there was a significant decrease in fluorescence values of each calibrator as compared to the values obtained at the start of the test (Table 3).
Control of the assay
To demonstrate the accuracy of the method, the control of the assay was evaluated in all the storage temperatures studied. The stability of an analyte is commonly taken as the time in which a change of 20 % of its concentration occurs in respect with the original concentration [19]. The behavior of the control of the assay in respect to the expected concentration when stored at either -20 ± 2 °C, 23 ± 2 °C and 37 ± 2 °C, respectively, are shown in the figure.
The expected value of control was 84.20 ng/mL. The average concentration obtained when the UMELISA® TIR NEONATAL was experimentally stored at -20 °C was 80.02 ± 6.34 ng/mL. Notably, the %RA was within the established range during the whole study.
The behavior was different when evaluating temperatures of 23 ± 2 ºC and 37 ± 2 ºC. In both cases, an important drop in the IRT values is observed when the reagent kits are stored for more than four days at room temperature and two days at 37 ºC. The mean concentration values obtained in these cases were 61.77 ± 11.56 ng/mL and 46.83 ± 8.12 ng/mL, respectively. Correspondingly, the %RA calculated for these conditions were outside the limits established for this type of test (Range ± 20 %).
DISCUSSION
Good storage practices for biological materials is an essential component of any laboratory. Biological samples often degrade over time when stored at room temperature, but some samples may also lose integrity at low temperatures if subjected to multiple freeze-thaw cycles. The best storage temperature for a given biological sample or reagent often varies according to the type of biological material, the solution in which it is suspended, the intended use of the sample and the time it will be stored. The most common storage temperatures are room temperature (20-23 ºC), refrigeration (2-8 ºC), freezing, deep freezing and cryogenic freezer storage.
Shelf life is commonly estimated using two types of stability tests: real-time stability tests and accelerated stability tests. In the former, a product is stored at recommended storage conditions and monitored until it fails the specification. On the contrary, in accelerated stability tests a product is stored under highly stressing conditions (temperature, humidity, pH). In both, degradation at the recommended storage conditions can be predicted using known relationships between the acceleration factor and the degradation rate.
The assessment of shelf life has evolved from examining the data and making an educated guess through plotting to the application of rigorous physical-chemical laws and statistical techniques. Regulators now insist that adequate stability testing should be conducted to provide evidence on the performance of a drug or a biopharmaceutical product at different environmental conditions and to establish the recommended storage conditions and shelf life [20-22]. Recently, Tsong reviewed the latest approaches to statistical modeling of stability tests [23] and ICH has published some guidelines for advanced testing design and data analysis [24-25].
Temperature should be tested taking into account the nature of the product and the recommended storage temperature. The selected temperatures should stimulate relatively fast degradation and quick testing but not enough as to destroy the fundamental properties of the product. In fact, it is not reasonable to test any product at very high temperatures for a very short period of time. Under those conditions, the mechanisms of degradation may be very different than those occurring at the recommended storage temperature. It is more advisable to choose the adjacent levels adequately so that degradation trends could be larger than experimental variability. Moreover, the chose levels depend on the nature of the product and its analytical accuracy, but other practical implications may be considered. For instance, testing should be performed at time intervals encompassing the target stability at each elevated temperature [11].
The low stability of IRT in dried blood spots on filter paper associated with temperature has been previously reported [26, 27]. According to the results obtained in our study, it was proven that storage -20 ± 2 ºC did not affected the stability of IRT during evaluation time, obtaining values of %R and %RA within the ranges established for this type of assay. These were in agreement with reports by Li et al. [16]. In the case of the temperature of 23 ± 2 ºC, although the quality parameters and the functional characteristics of the calibration curve remained within the established ranges for 14 days, the accuracy of the test control was affected from day 6, showing values of %RA above 20 %. In a similar study conducted in Wisconsin, it was reported that the concentration of IRT in dried blood samples stored at room temperature for 24 h decreased by 2 % [28].
Regarding the storage temperature of 37 ± 2 ºC, a sudden drop in the fluorescence values of each calibrator is observed from the second day after the beginning of the test. Concentration of the assay control is also affected. Since degradation of a biological product is usually defined in terms of loss of activity or performance, this is considered to be degrading when any characteristic of interest decreases (for instance, potency or performance).
Degradation usually follows a specific pattern depending on the kinetics of the chemical reaction. The degradation rate depends on the conditions where the chemical reaction takes place. Products degrade faster when subjected to acceleration factors such as temperature, humidity, pH and radiation. Temperature is probably the most common acceleration factor used for testing chemical, pharmaceutical and biological products. This is related to its association to the degradation rate, which relationship is well characterized by the Arrhenius equation [11]. In respect to IRT, there are evidences on a 40 % decrease of the concentration of this analyte in just one week in a dried blood sample stored at 27 °C and 80 % humidity [28].
Here were found negative effects on the on the stability of dried blood calibrators and control of the UMELISA® TIR NEONATAL during storage at 23 ± 2 ºC and 37 ± 2 ºC. These could lead to a decreased accuracy of the method, since lower fluorescence values of the calibrators can lead to the overestimation of the samples evaluated, leading to the subsequent increase in the false positive results of the test.
Remarkably, it is very important to comprehend the susceptibility of markers in dried blood to degradation, when exposed to stressing temperature and humidity conditions, in order to maintain the integrity of the sample for high quality measurements. In this line, it is recommended that assay calibrators and control should receive maximum protection against potential degradation caused by their storage conditions and transport environments.
CONCLUSIONS
According to the results obtained in this study, the quality parameters of the UMELISA® TIR NEONATAL are affected according to their storage conditions. It was demonstrated that the temperature directly influences the analytical performance of the UMELISA® TIR NEONATAL. It was also shown that the kit can be exposed to a temperature of -20 ± 2 ºC for 20 days and a temperature of 23 ± 2 ºC, for 14 days, without affecting its performance. Noteworthy, it is not advised to store kit components at 37 ± 2 ºC for more than 4 days, since its optimum quality parameters are affected.
REFERENCES
1. Parad RB, Comeau AM. Newbornscreening for cystic fibrosis. Pediatr Ann. 2003;32(8):528-35.
2. Sontag MK, Hammond KB, Zielenski J, Wagener JS, Accurso FJ. Two-tiered immunoreactive trypsinogen-based newborn screening for cystic fibrosis in Colorado: screening efficacy and diagnostic outcomes. J Pediatr. 2005;147(3 Suppl):S83-8.
3. Riordan JR, Rommens JM, Kerem B, AlonN, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066-73.
4. Massie RJ, Curnow L, Glazner J, Armstrong DS, Francis I. Lessons learned from 20 years of newborn screening for cystic fibrosis. Med J Aust. 2012;196(1):67-70.
5. Dijk FN, Fitzgerald DA. The impact of newborn screening and earlier intervention on the clinical course of cystic fibrosis. Paediatr Respir Rev. 2012;13(4):220-5.
6. Farrell PM, Kosorok MR, Laxova A, Shen G, Koscik RE, Bruns WT, et al. Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. N Engl J Med. 1997;337(14):963-9.
7. Grosse SD, Rosenfeld M, Devine OJ, Lai HJ, Farrell PM. Potential impact of newborn screening for cystic fibrosis on child survival: a systematic review and analysis. J Pediatr. 2006;149(3):362-6.
8. VanDevanter DR, Pasta DJ, Konstan MW. Improvements in lung function and height among cohorts of 6-year-olds with cystic fibrosis from 1994 to 2012. J Pediatr. 2014;165(6):1091-7 e2.
9. Crossley JR, Elliott RB, Smith PA. Dried-blood spot screening for cystic fibrosis in the newborn. Lancet. 1979;1(8114):472-4.
10. González EC, Castells EM, Frómeta A, Arteaga AL, Del Río L, Tejeda Y, et al. SUMA technology and newborn screening tests for inherited metabolic diseases in Cuba: An Overview of the First 30 Years. J Inborn Errors Metab Screen. 2016;4:1-9.
11. Magari RT. Assessing shelf life using real-time and accelerated stability tests. BioPharm Int. 2003;16 (11):36-48.
12. Levy HL, Simmons JR, MacCready RA. Stability of amino acids and galactose in the newborn screening filter paper blood specimen. J Pediatr. 1985;107(5):757-60.
13. Waite KV, Maberly GF, Eastman CJ. Storage conditions and stability of thyrotropin and thyroid hormones on filter paper. Clin Chem. 1987;33(6):853-5.
14. Zytkovicz TH, Eaton RB, Foley TP, Lister BC, Rojas DA, Schwerier ME. Instability of enzymes, antibodies, and other analytes in dried blood spots - is the major problem heat or humidity. In: Therell BL, Aldis BG, editors. Proceeding of the Eleventh National Symposium on Neonatal Screening; Sept. 12-16, 1995; Corpus Christi, Texas. Washington, DC: ASTPHLD; 1995. p. 293-5.
15. Freer DE. Observations on heat/humidity denaturation of enzymes in filter-paper blood spots from newborns. Clin Chem. 2005;51(6):1060-2.
16. Li L, Zhou Y, Bell CJ, Earley MC, Hannon WH, Mei JV. Development and characterization of dried blood spot materials for the measurement of immunoreactive trypsinogen. J Med Screen. 2006;13(2):79-84.
17. Castells Martinez EM, Gonzalez EC, Tejeda Y, Frometa A, Martin O, Espinosa M, et al. An Enzyme Immunoassay for Determining Immunoreactive Trypsinogen (IRT) in Dried Blood Spots on Filter Paper Using an Ultra-Microanalytical System. Appl Biochem Biotechnol. 2018;186(4):1034-46.
18. Ochoa R, Martínez JC, Ferriol X, García AM, Estrada E, Blanco R, et al. Principios y procedimientos para la validación de inmunoensayos cuantitativos empleados para evaluar la inmunogenicidad de vacunas. Vaccimonitor. 1999;8(10):9-13.
19. Frómeta-Suárez A, Marrero-González N, González-Reyes EC, Lugo-Vallejo E, Fernández- Fernández L, Doménech- Mylnikova MB. Estudio comparativo de los tres papeles de filtro en los ensayos UMELISA para el tamizaje neonatal de hipotiroidismo congénito y fenilcetonuria. Rev Biomed. 2002;13(4):241-7.
20. FDA. Guidelines for submitting documentations for the stability of human drugs and biologics. Rockville (MD): Food and Drug Administration; 1987.
21. ICH. Stability testing for new drug substances and products, Q1A(R). Geneva: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 1994.
22. ICH. Stability testing for new drug substances and products, Q1A(R2). Geneva: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 2003.
23. Tsong Y. Recent issues in stability testing. J Biopharm Stat. 2003;13(3):7-9.
24. ICH. Stability data evaluation, Q1E. Geneva: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 2003.
25. ICH. Bracketing and matrixing designs for stability testing of new drug substances and products. Q1D. Geneva: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 2003.
26. Cabrini G, Pederzini F, Perobelli L, Mastella G. An evaluation of an enzyme immunoassay method for immunoreactive trypsin in dried blood spots. Clin Biochem. 1990;23(3):213-9.
27. Dhondt JL, Farriaux JP. What do immunoreactive trypsin assays measure? Screening. 1994;3(1):33-8.
28. Kloosterboer M, Hoffman G, Rock M, Gershan W, Laxova A, Li Z, et al. Clarification of laboratory and clinical variables that influence cystic fibrosis newborn screening with initial analysis of immunoreactive trypsinogen. Pediatrics. 2009;123(2):e338-46.
Received in July, 2018.
Accepted in September, 2018.
Elisa M Castells-Martínez. Neonatal Screening Laboratory, Immunoassay Center. 25th Ave and 134 Street, Cubanacán, Playa, CP 6653, Havana, Cuba. E-mail: elisa.castells@cie.cu.














