Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Plantas Medicinales
versión On-line ISSN 1028-4796
Rev Cubana Plant Med vol.18 no.2 Ciudad de la Habana abr.-jun. 2013
ARTÍCULO ORIGINAL
Quantification, chemical and biological characterization of the saponosides material from Sida cordifolia L. (escobilla)
Cuantificación, caracterización química y biológica del contenido saponósido de Sida cordifolia L. (escobilla)
Biol. Oscar Julián Velásquez Ballesteros, MSc. Elizabeth Murillo Perea, Dr. John Jairo Méndez, Dr. Walter Murillo Arango, Biol. Diana Alexandra Noreña
Universidad del Tolima. Colombia.
ABSTRACT
Introduction: Sida cordifolia L. (Malvaceae) is a weed about which not much is known in Colombia. This plant is used in folk medicine to treat oral mucosa, blennorrhea, asthma and bronchitis. In Brazil it finds application as an anti-inflammatory, while in Colombia its "baba" is used for treating hair loss, constipation and internal fever, among other ailments.
Objectives: to quantify the saponoside content and evaluate its antioxidant and antifungal functionality.
Methods: we prepared organic, aqueous and hydroalcoholic extracts from the aerial section of the plant. The saponoside material was quantified by the DNS and p-anisaldehyde methods. The most concentrated extracts were selected for antioxidant and antifungal assays.
Results: it was found that Sida cordifolia, collected in Ibague-Colombia, is a good source of saponins with diverse chemical structures, mainly of steroidal nature, some of which may be hecogenin, diosgenin or a homologue.
Conclusions: these factors may contribute, at least in part, to the antioxidant and antifungal functionality of Sida cordifolia L., but this capacity may be modified if these saponins act independently or together with some other metabolites of the plant such as tannins, flavonoids steroids, and alkaloids among others.
Key words: Sida cordifolia, saponins, weed, antioxidant, p-anisaldhyde.
RESUMEN
Introducción: Sida cordifolia L. (Malvaceae) es una hierba de la cual se tiene un conocimiento limitado en Colombia. Se utiliza en la medicina popular para el tratamiento de la mucosa oral, la blenorragia, el asma y la bronquitis. En Brasil encuentra aplicación como antiinflamatorio, mientras que en Colombia su "baba" se utiliza para tratar la pérdida del cabello, el estreñimiento, y la fiebre interna, entre otros padecimientos.
Objetivos: cuantificar el contenido saponósido y evaluar sus propiedades antioxidantes y funcionalidad antifúngica.
Métodos: se prepararon extractos orgánicos, acuosos e hidroalcohólicos de la parte aérea de la planta. El material de saponósidos se cuantificó por los métodos del DNS (dinitrosalicylic acid reagent) y de p-anisaldehído. Los extractos más concentrados se seleccionaron para los ensayos antioxidante y antifúngico.
Resultados: estos mostraron que Sida cordifolia, colectada en Ibague-Colombia, es una buena fuente de saponinas con diversas estructuras químicas, principalmente de naturaleza esteroidal, algunas de las cuales pueden ser hecogenina, diosgenina o un homólogo.
Conclusiones: estos factores pueden contribuir, al menos en parte, en la funcionalidad antioxidante y antifúngica de Sida cordifolia L., pero su capacidad se puede modificar si esas saponinas actúan de forma independiente o en conjunto con otros metabolitos de la planta, como taninos, esteroides flavonoides, alcaloides, entre otros.
Palabras clave: Sida cordifolia, saponinas, hierba, antioxidante, p-anisaldehído.
INTRODUCTION
Specialized botany identifies a group of medium sized plants (50-70 cm), such as arvense, brushwood or weed, plants which grow with considerable vigor and great ability to spread in areas controlled by humans, because in most cases they are endemic species highly adapted to the environment. They can establish competition with improved pastures in terms of living space, nutrients, water, sunlight and CO2.1
The scientific world has shown scarce interest for those plants. Attention has been generally oriented to macro-morphological description2-4 or to counting the weeds that accompany beneficial commercial crops species,1,5 many of wich are useful in many parts of the world as food (Arctium lappa), herbal medicine (Taraxacum officinale) and a large number of them as ornamentals.6 Recent studies have focused on some weeds as a sources of secondary metabolites with pharmacological,7 antimicrobial8 and antioxidant uses.9
Sida cordifolia L. (Malvaceae) is a weed about which not much is known in most Latin American countries including Colombia. Commonly called malva blanca, sánalo todo (Argentina), escobilla, escoba babosa (Colombia), puchana, sinchi pichana (Peru), escoba acorazonada (Puerto Rico), escobillo, escoba negra (Spain) and bala (India), it is a widespread species throughout the tropics (North, Central and South America), and is also prevalent in Africa, Asia and Oceania.
This plant is used in folk medicine to treat oral mucosa, blennorrhea, asthmatic bronchitis and nasal congestion.10 In Brazil finds application as an anti-inflammatory,11 while in Colombia its "baba" is used for treating hair loss, constipation and internal fever, among other ailments. Some studies have shown the presence in its leaves of sympathomimetic amines and alkaloids such as ephedrine, pseudoephedrine, vasicinone and vasicine.12 The antioxidant capacity of the plant and the use of aqueous extracts from its leaves for liver regeneration have been revealed.13,14
Furthermore, saponins are a specific class of secondary metabolites widely distributed in the plant kingdom. They are constituted by a triterpenic or steroidal skeleton attached to one (monodesmosidic) or more (bidesmosidic) sugar chains. These chemical compounds are of various biosynthetic origins, hence their great structural diversity. It is estimated that more than half of land plants contain saponins.15 Their biological and pharmacological functionality (hemolytic, anti-tumor, anti-inflammatory, molluscicidal, etc.) is a consequence of their chemical diversity.16
This study was carried out in compliance with biodiversity rights, using the aerial section as a raw material to prepare aqueous and hydroalcoholic extracts. The saponoside material was quantified by two spectrophotometric methods. The most concentrated extracts were selected and antioxidant activity was evaluated with the purpose of correlating this biological functionality with the saponin content of the plant.
METHODS
Collection, sample conditioning and extract preparation
Plant material (leaves and inflorescences) was collected in optimum phytosanitary and vegetative development condition (August 16, 2010) in the Ibagué suburban zone (22 ºC, 57 % RH) and identified at the TOLI herbarium in Tolima University (Colombia) with the 7083-reference code. The sample was cleaned, dried (48 h, 45 ºC), crushed and degreased (Soxhlet, n-hexane). This treated material was stirred mechanically (5 h), using water, methanol, methanol-water (95:5), ethanol-water (95:5) and ethanol (1 g:10 mL plant/solvent) until the sample was depleted. Crude extracts were filtered and concentrated at reduced pressure in a Bûchi R114 rotary evaporator and stored (4 ºC). Extracts were identified as: Aqueous extract (AE), hydromethanolic extract (HME), methanol extract (ME), hydroethanolic extract (HEE) and ethanolic extract (EE).
Preliminary assays for saponins recognition
The presence of saponosides in extracts was verified with several scientifically recognized qualitative tests for preliminary evidence of secondary metabolites: the foam test (based on the surfactant capacity of saponins), the Rosenthaler test (violet colors are obtained with pentacyclic saponins) and the Liebermann-Burchard test (pink or red colors for triterpenic genins and blue or green for steroidal).
These tests were supplemented by a thin layer chromatography analysis (TLC). Different chromatographic conditions were tested, but the best ones were silica gel 60 F-254 as stationary phase and chloroform/ethanol/water (8:2:0.5) as eluent. To visualize the spots, plates were sprayed with various chromogenic agents (p-anisaldehyde, vanillin, antimony trichloride, silver nitrate and iodine vapors) and put on heat. Digitonin, hecogenin, diosgenin, cholesterol, and glucose were used as positive reference standards (1 mg/mL).
Presence of the carbohydrate fraction attached to the genins was evident on 60 F-254 chromatography plates (stationary phase) and ethyl acetate/acetic acid/methanol/water (10:4:4:2) as mobile phase. Spots were visualized by spraying a diphenylamine/aniline/phosphoric acid/acetone mixture (4 g:4 mL:20 mL:200 mL).
Phytochemical screening
Phytochemical screening was performed with the ethanolic extract of the plant using Molish (carbohydrates), foam, Rosenthaler, hemolysis (saponins), Folin-Ciocalteu (polyphenols), chloride ferric salt gelatin (tannins), ammonia vapors, Shinoda (flavonoids), Arnow (phenylpropanoids), Bornträger (anthraquinone), Lieberman-Burchard, Salkowski (terpenes/steroids), vanillin/HCl (iridoids), Dragendroff, Mayer, Wagner, Tanred, Erhlic, Reineckato, Valser (alkaloids), Baljet, Kedde, m-dinitrobenzene (cardiotonic agents), NaOH/heat/UV light (coumarins), m-dinitrobenzene, Raymond, Mathoud and CCD (terpene lactones) tests. The crosses system was used to specify qualification of secondary metabolites.
Indirect quantification of saponosides
Prior analytical determination, an acid hydrolysis process with HCl 2.5 N was performed (95 ºC, 3h with constant agitation). The hydrolyzed material was neutralized with sodium bicarbonate and the Dinitrosalicylic Acid reagent (DNS) was combined with each extract (1:1). The mixture was vigorously stirred, heated in a water bath (100 ºC, 5 min) and cooled in an ice-water bath. Distilled water was then added (5 mL), and absorbance was read at 540 nm in a Helyos-Gama spectrophotometer and interpolated on a calibration curve prepared with glucose (50-1600 µg/mL).17
Direct quantification of saponosides
The methodology followed was the one proposed by Hata et al.,18 with some modifications: 2 mL of each extract (AE, HME, ME, HEE, EE), 1 mL of A reagent (p-anisaldehyde 0.5 % in ethyl acetate) and 1 mL of B reagent (H2SO4 in 50 % ethyl acetate) were mixed and homogenized in a vortex. The mixture was immediately heated in a water bath (60 ºC, 20 min), and then each tube was placed in a room temperature water bath and protected from light during the reaction. Optical density of samples was determined at 430 nm and interpolated into a calibration curve using digitonin (20-1 200 µg/mL).
Structural identification of saponins
For this purpose, each sample was taken to total dryness and mixed with two drops of SbCl3 in 60 % perchloric acid. Digitonin, hecogenin, diosgenin and 60 % HClO4 in the absence of SbCl3 were used as controls.
Spectrophotometric analysis
Infrared spectra of samples were performed in Nicolet FT-IR 380 equipment. The UV-VIS spectra were measured in an Evolution 600 spectrophotometer, with 1 cm cell width, and a wavelength between 380-700 nm.
Hemolytic activity
Human erythrocytes (O+) were collected with sodium citrate, washed twice with isotonic phosphate buffer (10 mM, pH 7.4) and centrifuged (500 g x 5 minutes). The pellet was resuspended to 40 % with the same solution. 500 µL of each sample and 9 µL of cell suspension were placed in a vial and incubated at 37 ºC for 20 minutes. The samples were then centrifuged (500 g x 5 minutes) and the supernatant transferred to a quartz cell. The free hemoglobin amount was spectrophotometrically determined at 540 nm.19 An isotonic solution (150 mM NaCl) was used as a basal hemolysis control and Tween 20 as a maximum absorbance control. The hemolysis percentage was calculated using the equation: Hemolysis percentage = [(Asamle-ANaCl)/(Atween-ANaCl )]*100
Antioxidant activity
2,2-diphenyl-1-picrylhydrazyl radical stabilizer activity (DPPH)
Samples were placed on silica gel chromatography plates (60 F-254) using a chloroform/ethanol/water (8:2:0.5) mixture as mobile phase. A 0.2 % radical solution was used as chromogenic agent. The same procedure was performed in parallel, but spraying the plate with sulfuric acid/acetic acid/water (80:10:10), exclusively to reveal saponins.
2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) cation radical inhibitory activity (ABTS*+)
Plant material was dissolved in phosphate buffer (2 mL, 50 mM, pH 7.5) and ethyl acetate (5 mL). The mixture was mechanically stirred and transferred to a separatory funnel. The solid residue (totally colorless) was discarded. The aqueous and organic phases were collected separately to evaluate respectively the hydrophilic and lipophilic activity of the plant.20
ABTS*+ radical (7 mM) was prepared with ammonium persulfate (4.9 mM) mixed 1:1. The mixture was kept in the dark (16h) and then diluted with ethanol (96 %) until reaching 0.7 absorbance (± 0.02) at 754 nm (maximum absorption wavelength). Sample was added (100 µL) to 3.9 mL of radical with a quick stir and the change in optical density was measured at 754 nm during 8 minutes.21 Absorbance values were interpolated in a calibration curve prepared with ascorbic acid (50-500 µg/mL). ABTS*+ stabilization capacity was determined using the equation ABTSSC= [AABTS.+- A8min/ A ABTS.+]×100
Where:
ABTSSC: ABTS radical stabilization capacity, expressed as a percentage
AABTS*+: ABTS absorbance before adding the sample
A8min: absorbance of mixture at 8 minutes
The functionality of samples was compared with that of BHT (Butylated hydroxytoluene) [10, 15 and 20 µL/mL] and quercetin [50 µL/mL].
Reducing power measurement
Reducing power was determined following the methodology described by Pérez et al.,22 with some modifications. One milliliter of each extract was mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6), potassium ferricyanide (2.5 mL, 1 %), and incubated at constant temperature (50 ºC, 20 minutes). Trichloroacetic acid (2.5 mL, 10 %) was then added. The mixture was centrifuged (3 500 r.p.m., 10 min). A supernatant aliquot was dissolved in an equal amount of distilled water and ferric chloride was immediately added (0.5 mL, 0.1 %). Absorbance was determined at 700 nm, using ascorbic acid as a positive control (50 and 100 µg/mL). In this assay, the reducing power of the extracts was directly related to absorbance values.
Total antioxidant capacity (TAC)
A reagent was prepared by mixing H2SO4 (0.6 M), Na3PO4 (28 mM) and (NH4).Mo7.4H2O in a 1:1:1 proportion. 3 mL were mixed with 0.3 mL of each extract and incubated at 95 ºC for 90 minutes. Absorbance was measured at 696nm and the results were expressed as milligrams of ascorbic acid equivalents per gram of dry material (AA Emg/DMg).23
Elisa test
Before measuring in vitro susceptibility of Fusarium oxysporum and Rhizopus oryzae, a smear of each colony was transferred to test tubes that contained sterile distilled water (10 mL). 5 x 106 colony forming units were counted with an Improved Neubauer chamber and used in each case. 100 µL of Sabouraud liquid agar were mixed with a sample aliquot (12 to 50 µL) and enough water to obtain 200 µL in order to reach concentrations of 1 000, 2 000, 4 000, 6 000 and 8 000 (µg/mL). Each treatment was performed 4 times and absorbance measured at 490 nm during 5 days in an Elisa reader (ELX800G DIAREADER).24 Digitonin, diosgenin and hecogenin (2500 mg/mL) were used as standards.
Statistical analyses
All data, except those from the Elisa test (n= 4 ± SD), are expressed as the mean of three determinations (n= 3 ± SD). A Kolmogorov-Smirnov test was used to determine the normal distribution of absorbance values, and a linear regression analysis was performed to validate both quantification methods using two statistical programs: STATGRAPHICS Centurion XV 15.2.05 version and Info Stat student version. Data from the quantification of saponoside material and about antioxidant and antifungal activity were subjected to linear regression, variance and principal component analysis. In all cases the correlation between variables was verified with a 95 % significance level (p£ 0.05).
RESULTS
A transverse cut of the leaves from Sida cordifolia revealed an anomocytic stomata pattern in which guard cells are undifferentiated from each other in shape and size and are not surrounded by any subsidiary cell (common in Cucurbitaceae, Malvaceae, Tamariaceae and Papaveraceae). Primary xylem with metaxylem (with thick helicoidal elements) branched unicellular trichomes and glandular pili were also found.
Some pharmacognostic indexes determined in the Sida cordifolia aerial section (leaves and flowers) revealed 37.3 ± 0.09 % of dry matter, 10.9 ± 0.09 % of ash, dark green and brown extracts with fresh grass smell, 0.8 to 0.98 (g/cm3) density, and total solids between 1.12 and 2.2 %.
Results obtained from qualitative tests performed for recognition of the presence of saponins in Sida cordifolia organic extracts are shown in Table 1.
The table above shows that methanol (ME) and hydromethanolic (HME) extracts had the most outstanding responses in all assays. It is important to mention that foaming activity is higher in those bidesmosidic saponins, and this property may increase with the glycosidic chain length in monodesmosidic saponins. The table also shows the highest hemolysis percentages for HME and ME, and the best eluent selected for thin layer chromatography.
Phytochemical screening
Phytochemical analysis revealed the diversity of secondary metabolites, such as polyphenols, tannins, flavonoids, phenylpropanoids, anthraquinones and alkaloids, among others. As was expected, tests for saponins, terpenes/steroids and reducing carbohydrates were unquestionable.
Thin layer chromatographic analysis (TLC)
A chromatography plate revealed with p-anisaldehyde/sulfuric acid/acetic acid and heat (0.5:9:0.5:0.1 mL, 10 min, 100 °C) showed abundant spots (0.3 -0.7 RFs), including some violet and purple hues. Using vanillin as chromogenic agent (2 % in ethanol, 5 min, 90 °C) and spraying again with acetic anhydride/sulfuric acid (12:1; 85-90 °C), revealed yellow-gray and blue-violet spots (indicative of saponins). Meanwhile, the plate revealed with silver nitrate in acetone (12:400 mL), dried and sprayed with NaOH (0.5 M in ethanol), showed reducing sugars (dark brown spots on a light background). Another plate was incubated in a glass chamber saturated with iodine vapor. In this case saponins appeared as yellow-brown spots. Steroidal saponins were observed at visible as yellow spots on a white-cream background. When a plate was sprayed with antimony trichloride (10 % in chloroform, 70-90 °C, and 40 min), saponins were observed as blue fluorescent spots at UV light.
Specific chromatographic analysis for carbohydrates showed stains with similar to those from reference standards, and enabled identification of glucose, arabinose, galactose (carbohydrates commonly found associated with saponins), fructose and sorbose (reported as companions of saponins in plants).
Identification of saponins
Some reagents, such as SbCl3, form specific stainings when reacting with saponins (with some functional groups of the steroidal nucleus). HME and ME were used as models for identifying the structure of saponins. Table 2 shows the typical stainings obtained in each sample and the standards.
Spectrophotometric analysis
As a complement to chromatographic analysis, IR and UV-VIS spectra were determined for ME and HME extracts and some standars. In IR spectra, several bands were observed from -OH valence vibration (3 400 cm-1), C=O and C=C groups (1 550-1 700 cm-1), C-O-C asymmetric tension (1 050 cm-1), and pyran and furan tensions.
For the UV-VIS spectrum, spectrophotometric scanning from 380 to 700 nm showed the highest absorbance at 430 nm ± 2 in all cases.
Quantification of saponosides
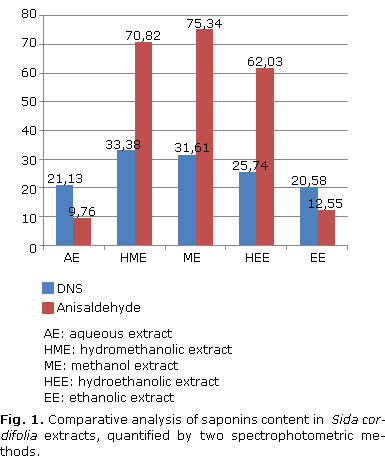
The following equations: Abs= 0.000508865*C-0, 0168209 (R2 = 99.947) for DNS and Abs= 0.000417649*C+0.110798 (R2= 98.6459) for p-anisaldehyde, were the basis for the quantification of saponoside material. Figure 1 was developed to compare saponin concentrations.
Hemolytic action of saponins
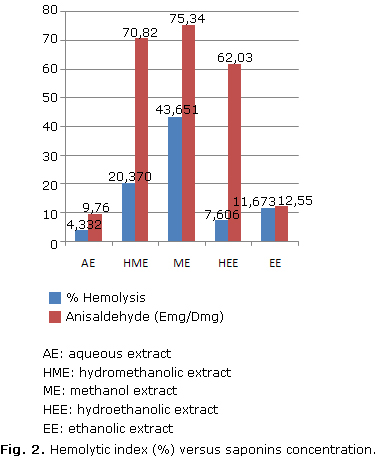
On the basis of the values obtained, a comparative analysis was carried out of the hemolytic index and saponosides content of extracts. The results are shown in Figure 2 reveals the results.
As shown in the figure, ME exhibits the highest hemolytic activity (44 %), followed by HME (20 %), EE (16 %), HEE (8 %) and AE (4 %). Except for EE, there is a direct correspondence with saponin content.
Antioxidant activity
Results from DPPH.+ radical discoloration were compared with the spots of another chromatography plate run under the same conditions, but revealed with p-anisaldehyde.
Violet spots generated from the saponoside material, and yellow-green stainings produced by antioxidant compounds were observed. There is a coincidence in some of the bands of both chromatography plates, revealing the highest concentration of compounds with antiradical activity against DPPH, mainly with low RFs (~0.3) and some high (~1.0).
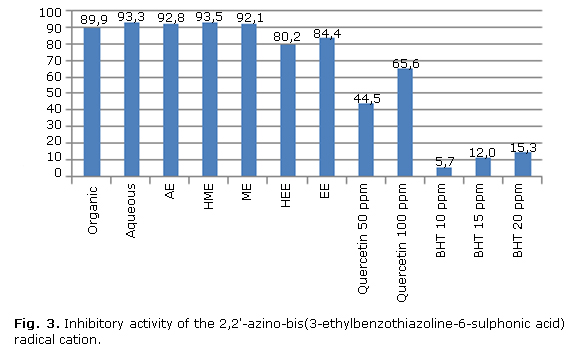
Performance of standards and extracts against ABTS radical is shown in Figure 3. A comparable action is observed in polar and non-polar phytocompounds (aqueous and organic phase) with respect to those present in ME and HME, which are in turn more active than BHT and quercetin.
The figure shows that organic (89.9 %) and aqueous phase (93.3 %), aqueous (92.8 %), hydromethanolic (93.5 %), methanolic (92.1 %), hydroethanolic (80.2 %), and ethanolic extracts (84.4 %) had inhibition percentages above 50 %, being more active than, one of the synthetic antioxidants most widely used in the food industry, and even exceeded theactivity of quercetin, the most abundant natural flavonoid. Applied linear regression analysis yielded the following equation: ABTS= 77.2046-0.308919* [anisaldehyde] (p= 0.8765).
Another method used for measuring antioxidant activity was FRAP. In this assay methanol and ethanol, either pure or mixed with water (HME, HEE), can remove from the plant compounds with greater reductant ability than that of ascorbic acid (50 and 100 µg/mL). The ratio between FRAP and saponins content is expressed by the equation: Reducing power = 0.714907-0.00300223*anisaldehyde (p= 0.7698).
The TAC variability of ascorbic acid prepared at different concentrations (40-640 mg/mL) and used as reference, was expressed by the calibration curve: Absorbance=0.003839[AA]-0.16805. HEE, EE and ME action seems comparable to the antiradical activity evidenced by themselves.
Antifungal activity
No activity against F. oxysporum and R. oryzae was observed in the extracts. However, some of their fractions showed activity in all the concentrations tested (data not shown).
DISCUSSION
The micro morphological characteristics observed are consistent with those described for the Malvaceae family, which immediately allows placement of Sida cordifolia in a specific taxon based on some specific physical parameters. The pharmacognostic indexes determined are an indication of the expected content of bioactive ingredients in each extracts. The dry matter content leads to pondering the capacity of the plant to store a considerable amount of water (62.7 ± 0.9 %), although the collection site is located on the edge of a road, with low humidity, high air flow, high temperature and plenty of light. This is an "edge effect" which directly affects the physiology of the plant, forcing it to respond to stressful conditions by storing a lot of water as a survival mechanism.
The scientific world acknowledges the presence of saponins mainly through the foam test and hemolytic index. The former is based on the persistent, abundant and relatively stable foam appearing when aqueous solutions are shaken, which results from the ability of these compounds to decrease water surface tension and form micelles as detergents do.25
Secondary nuclei evidence from phytochemical screening could be a consequence, partial as it may be, of the environmental stress to which the plant is subjected due to the high anthropogenic impact on its ecological niche (near a national road), and would justify some of the ethnomedical uses given to the plant: bronchial disorders, aphrodisiac and central nervous system stimulant (alkaloids), slimming and syphilis treatment (saponins), and external wound healing because of its flavonoids.14,26
Chromatographic analysis showed that chemical constituents exhibiting high foaming activity, increased hemolytic index and the highest reliability in TLC are to be found in ME and HME. Use of some reference standards for sapogenins and carbohydrates not only confirmed previous assays, but also suggested that the plant contains steroidal saponins, some of which may be hecogenin, diosgenin or a homolog of them.
The carbohydrates attached to the sapogenins from Sida cordifolia are probably those commonly reported for other saponins. It should be noted that the number of carbons attached to the genina may be in a 1:1 ratio, but this is not the most frequent situation. The sugar portion of the glycoside is generally constituted by 1-2 linear or branched oligosaccharides, the molecule may contain up to 11 carbons (3 to 5 is common), and the link between the carbohydrate and the sapogenin may be either ether or ester.27
Some authors affirm that a red color (Table 2) is associated with the D5 double bound of 25a or 25ß-3: 5-spirostan diene sapogenins. Loss of that double bond is directly related to red colour disappearance.28
Besides absorption bands characteristic of steroidal substances (3650-3590 and 1055 cm-1 for -OH bonded to a secondary carbon, 2960-2850 and 1485-1445 cm-1 for -CH stretching and -CH2 bending, etc), the spectroscopic analysis also revealed several bands generated from C-O pyran and furan rings tensions, which are located around 850, 900, 920 and 987 cm-1 (steroidal saponin fingerprint).
The presence of phytosterols seems to constitute interference in the IR spectrum and greatly reduces the intensity of bands, preventing their occurrence or shifting their position, for example the 1125 cm-1 band, a fact not observed in standards (pure compounds). On the other hand, the band observed in all extracts at UV (430 nm ± 2) is typical of steroidal saponins.29,30 However, rings A and B, their substituents and stereochemistry, play no role in chromophore training or in maximum absorption (ëmax), due to saturated sapogenins, such as tigogenin or gitogenin, which react similarly to hecogenin acetate, yonogenin and tokorogenina triacetate, among others. Additionally, the hydroxyl groups at 3 position seem to participate in the absorption process of UV radiation.31
Results in Figure 3 clearly show that water was less able to extract the metabolite of interest, while methanol or its mixture with water showed greater extracting ability. It is also observed that the DNS seems to be less sensitive than the p-anisaldehyde method. According to some authors this last spectrophotometric method allows to determine all sapogenins, regardless of their structure.18,31,32
The low correlation observed between saponosides content and hemolytic activity has its origin in the biochemical nature of the saponins (steroidal and triterpene), the number of glycosidic substituents (monodesmosidic, bidesmosidic, etc.), and their degree of complexity, as well as blood type and Rh factor. Therefore, those with the highest hemolysis percentages may have a higher ratio of monodesmosidic steroidal saponins (more active) than those with low percentages, which may have both steroidal and triterpenic bidesmosdic types.25,31
Numerous biological effects of saponins have been justified by its action on the cell membrane. It is believed that the injuries caused by these metabolites result from the formation of micelles arising from their interaction with cholesterol on the membrane plane, a bond which is possibly made between the hydrophobic fraction of saponin and cholesterol located on the outside perimeter. To achieve that union, the saponin molecule must dispose as a ring for others. However, the interaction may be more complex.25
TLC against DPPH radical test results indicate that S. cordifolia has a rich content of antioxidant compounds, mostly saponins. On the other hand, the ABTS*- test determines the capacity of saponins to decrease the potential energy of radicals in an aqueous medium. In this way the ABTS*- assay gives credence to the determination of the antioxidant capacity of S. cordifolia, indicating that the results observed through the DPPH qualitative assay are not due to confusion or misperceptions. It should be noted that the chromophore formed was read at 754 nm, not interfering with the absorption of the complex formed by the saponins and p-anisaldehyde reagents (430 nm ± 2).
The moderate antioxidant activity expressed by the saponins from S. cordifolia against ABTS*- could result from the fact that this radical is thermodynamically reduced by reducing compounds which have a lower redox potential than itself (0.68 V), as is the case with phenols.33 In turn, the FRAP assay cannot detect compounds that act by transferring H+, particularly thiols and proteins. In this way, the FRAP test becomes a reasonable procedure to measure the ability to maintain a redox status in cells or tissues.34 It should be noted that although there may be several compounds with antioxidant properties in a plant sample, each one exhibits its own activity and kinetic characteristics, depending on the presence of one, two or more kinds of components.
Measurement of the entire buffer system capacity of a natural product can give an idea of the antioxidant joint action of a pro-oxidant system against oxidative stress. The response depends on the fluid, tissue or cell studied. It must be born in mind that each environment has different antioxidant systems and different combinations (heterogeneous, an important, determining factor when results are evaluated and analyzed.35
Results indicate that antioxidants in the plant contribute differently to total antioxidant capacity, depending on the method applied. Regression analysis of the correlated variables did not show a good fit to the linear model, which suggests that the participation of saponins is low, in contrast with the joint action of metabolites (flavonoids, phenylpropanoids, tannins, alkaloids, etc.). In order to supplement the knowledge about antioxidant activity, a principal components analysis was performed (ABTS, CATH, reducing power, DNS, anisaldehyde and hemolysis), which revealed that only CATH is correlated with saponin content.
Some researchers interested in studying the biological activity of saponins from plants have found a moderate correlation between antioxidant activity and the total saponins fraction quantified by HPLC-DAD.16,34,36 This antioxidant functionality of S. cordifolia has also attracted the interest of a few scientists from different regions except Colombia.37,38
The chemical nature of components with antioxidant capacity is different, and so are their polar nature and their mode of action, which is also associated with molecular size, structural conformation, number, type and position of available substituents in the molecule, solubility characteristics, etc.39 It depends not only on the physicochemical characteristics of metabolites, but also on the way oxidation is induced and the assay type used.40
The low correlation between antioxidant activity and saponins content found in this study might be due, at least in part, to the size of saponin molecules, which would hinder their interaction with reagents, or perhaps the poor availability of nucleophilic groups (ex. -OH,-C=O) in their structure, which may be locked by forming glycosidic linkages, especially the carbonyl group (-C=O), whose presence increases the acidity of the hydrogen atoms attached to the alpha carbon, allowing them to be released as protons (H +) and stabilizing reactive oxygen species.
Although the antioxidant action of S. cordifolia is not fully revealed in the study, this activity is not viewed as negligible. Instead, it constitutes an incentive to continue proposing tests that shed light on the matter.
Results show that the aerial section of Sida cordifolia is a good source of saponins with various chemical structures, mainly of the monodesmosidic steroidal type, and its bioactivity could be modified if its saponins acted independently or in conjunction with the tannins, flavonoids, phenylpropanoids, anthraquinones and alkaloids of the plant.
The study also showed that the combination of FTIR with chromatographic techniques such as TLC, is a valuable tool to reveal the chemical nature of saponins from a natural product, leading to the development of a simple, rapid, specific and sensitive methodology for their study, adaptable to any laboratory conditions, mainly those with a limited availability of infrastructure. The study also revealed a promising future for Sida cordifolia, an unknown weed scarcely studied in Colombia, and constitutes the first research work into this topic conducted in our country.
REFERENCES
1. Sardiñas Y, Padilla C, Herrera RS, Torres, V, Noda A, Fraga N. Indicadores del crecimiento y desarrollo de Sporobolus indicus (L.) R. Br. (espartillo) en un agroecosistema de Panicum máximum vc. Likoni. Rev Cubana Ciencia Agrícola. 2010;44:421-5.
2. Guevara LI, Ramia M. Anatomía foliar de Panicum L., sección Parvifolia (Poaceae, Paniceae) en Venezuela. Rodriguésia. 2006;58(1):73-83.
3. Teillier S. Leucheria Landbecki (Phil.) Reiche (Asteraceae) es sinónima de leucheria runcinata D. Don. Rev Chilena Flor y Veg [serie en internet]. 2010 [citado 16 Ene 2012]; año 12(2). Disponible en: http://www.chlorischile.cl/leucheriaruncinataweb/leucheriaruncinata.htm
4. Marchessi JE, Subils R, Scaramuzzino RL, Crosta HN, Eseiza MF, Saint André HM, Juan VF. Presencia de Euphorbia davidii Subils (Euphorbiaceae) en la provincia de Buenos Aires: morfología y anatomía de la especie. Kurtziana. 2011;36:45-53.
5. Ordeñana DM, Tapia LJ. Comportamiento de arvenses en el cultivo de maíz (Zea mays L.) variedad NB-6, bajo dos sistemas de producción, convencional y orgánico en la finca El Plantel, Masaya 2008 [Trabajo de Grado]. Managua, Nicaragua: Universidad Nacional Agraria, Facultad de Agronomía, Departamento de Producción Vegetal; 2009.
6. Pérez ML, Nava RF. Plantas del estado de Querétaro con potencia para uso ornamental. Red de Revistas Científicas de América Latina y el Caribe, España y Portugal. 2007;24:83-115.
7. Murillo E, Viña A, Pérez CA, Ruiz VH. Actividad alelopática de las arvenses asociadas al cultivo de arroz (Oryza sativa L.) en el Tolima-Colombia. Información Tecnológica. 2006;17(2):15-24.
8. Toribio MS, Oriani DS, Skiliar MI. Actividad antimicrobiana de Centaurea solstitialis y Centaurea calcitrapa. Ars Pharmaceutica. 2004;45:335-41.
9. Gutiérrez DM, Ortiz CA, Mendoza AM. Medición de fenoles y actividad antioxidante en malezas usadas para alimentación animal [serie en internet]. 2008 [citado 6 Mar 2012) Simposio Metrología, 220(1018):1-5. Disponible en: http://www.cenam.mx/simposio2008/sm_2008/memorias/M2/SM2008-M220-1108.pdf
10. Franzotti EM, Santos CV, Rodrigues HM, Mourão RH, Andrade MR, Antoniolli AR. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca). J Ethnopharmacol. 2000;72:273-7.
11. Almeida JRGA, Silva-Filho RN, Nunes XP, Dias CS, Pereira FO, Lima EO. Antimicrobial activity of the essential oil of Sida cordifolia L. Rev Brasileira Farmacognosia. 2006;16:642-4.
12. Sutradhar RK, Rahman AM, Ahmad M, Bachar SC, Saha A, Guha SK. Bioactive alkaloid from Sida cordifolia Linn. with analgesic and anti-inflammatory activities. Iranian J Pharmacol Therapeutics. 2006;5:175-8.
13. Dhalwal K, Deshpande YS, Purohit AP, Kadam SS. Evaluation of the antioxidant activity of Sida cordifolia. Pharmaceutical Biology. 2005;43:754-61.
14. Lemos RL, de Melo GB, de Melo VA, Antoniolli AR, Teixeira PR, Zucoloto S, et al. Effect of the aqueous extract of Sida cordifolia on liver regeneration after partial hepatectomy. Acta Cirurgica Brasileira. 2006;21:37-9.
15. Gauthier C, Legault J, Girard-Lalancette K, Mshvildadze V, Pichette A. Haemolytic activity, cytotoxicity and membrane cell permeabilization of semi-synthetic and natural lupane-and oleanane-type saponins. Bioorganic Medicinal Chemistry. 2009;17:2002-8.
16. Sparg SG, Light ME, Van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004;94:219-43.
17. Casarrubias E. Estudio del efecto de la composición del medio de cultivo y de variables fisicoquímicas seleccionadas sobre la producción de invertasa por la levadura MPIIIa [Tesis doctoral]. Lardizábal, México: Instituto Politécnico Nacional Tepetitla de Lardizábal; 2005.
18. Hata Y, Reguero MT, de García LA, Buitrago G, Álvarez A. Evaluación del contenido de sapogeninas en variedades nativas de ñame (Dioscoreas sp.), provenientes de la colección de la Universidad de Córdoba. Rev Colombiana Ciencias Químico-Farmacéutica. 2003;32:149-57.
19. Zhu QY, Schramm DD, Gross HB, Holt RR, Kim SH, Yamaguchi T, et al. Influence of cocoa flavanols and procyanidins on free radical-induced human erythrocyte hemolysis. Clinical Developmental Immunology. 2005;12(1):27-34.
20. Arnao MB. Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case. Trends in Food Science Technology. 2000;11:419-21.
21. Marquina V, Araujo L, Ruíz J, Rodríguez-Malaver A, Vit P. Composición química y capacidad antioxidante en fruta, pulpa y mermelada de guayaba (Psidium guajava L.). Archivos Latinoamericanos de Nutrición. 2008;58:98-102.
22. Pérez RM, Vargas R, Martínez FJ, García EV, Hernández B. Actividad antioxidante de los alcaloides de Bocconia arborea. Estudio sobre seis métodos de análisis. Ars Pharmaceutica. 2003;44:5-21.
23. Abbasi MA, Raza A, Riaz T, Shahzadi T, Aziz-ur-Rehman, Jahangir M, et al. Investigation on the volatile constituents of Junglans regia and their in vitro antioxidant potential. Proceedings Royal Society Queensland. 2010;47:137-41.
24. Barile E, Bonanomi G, Antignani V, Zolfaghari B, Sajjadi SE, Scala F, et al. Saponins from Allium minutiflorum with antifungal activity. Phytochemistry. 2007;68:596-603.
25. Francis G, Kerem Z, Makkar HPS, Becker K. The biological action of saponins in animal systems: a review. British J Nut. 2002;88:587-605.
26. Jain A, Choubey S, Singour PK, Rajak H, Pawar RS. Sida cordifolia (Linn)-An overview. J Applied Pharmaceutical Science. 2011;1:23-31.
27. Bruneton J. Farmacognosia Fitoquímica Plantas Medicinales. Triterpenos y esteroides. Zaragoza-España: Acribia; 2001. p. 668-9.
28. Hardman NJ, Wright RJ, Phillips AD, Philip P. Structures, bonding, and reaction chemistry of the neutral organogallium (I) compounds (GaAr) n (n= 1 or 2) (Ar= terphenyl or related ligand): An experimental investigation of Ga-Ga multiple bonding. J Am Chemical Society. 2003;125:2667-79.
29. Garcia M, Castellanos MC. Evaluación del efecto sanitizante de un extracto biodegradable obtenido de la especie Solanum marginatum, de uso etnobotánico en Boyacá. Luna Azul. 2011;32:10-5.
30. Silverstein RM, Bassler GC, Morril TC. Infrared spectroscopy. Spectrometric identification of organic compounds. New York: John Wiley & Sons; 1991. p. 72-108.
31. Baccou JC, Lambert F, Sauvaire Y. Spectrophotometric method for the determination of total steroidal sapogenin. Analyst. 1997;102:458-65.
32. Fuentes JM, Jaramillo S, Rodríguez G, Rodríguez-Arcos R, Fernández J, Guillén R, et al. Effect of the extraction method on phytochemical composition and antioxidant activity of high dietary fiber powders obtained from asparagus by-products. Food Chemistry. 2009;116:484-90.
33. Navarro-Marfil R. Parámetros de calidad y componentes con interés nutricional del aceite de argán (Argania spinosa) [Tesis doctoral]. España: Facultad de Farmacia, Universidad de Granada; 2008.
34. Dini I, Tenore GC, Dini A. Saponins in Ipomoea batatas tubers: Isolation, characterization, quantification and antioxidant properties. Food Chemistry. 2009;113:411-9.
35. Escorza MAQ, Salinas JVC. La capacidad antioxidante total. Bases y aplicaciones. Rev Educación Bioquímica. 2009;28:89-101.
36. Gülçin I, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med. 2004;70:561-3.
37. Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, et al. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol. 2003;84:131-8.
38. Surveswaran S, Cai YZ, Corke H, Sun M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chemistry. 2007;102:938-53.
39. Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chemical Toxicology. 2006;44:198-206.
40. Puertas M, Hillebrand S, Stashenko E, Winterhalter P. In vitro radical scavenging activity of essential oils from Columbian plants and fractions from oregano (Origanum vulgare L.) essential oil. Flavour Fragance J. 2002;17:380-4.
Recibido: 30 de mayo de 2012.
Aprobado: 30 de diciembre de 2012.
Oscar Julián Velásquez Ballesteros. Departamento de Biología. Grupo de Investigación en Productos Naturales. Universidad del Tolima. Colombia. Correo electrónico: ojvb@live.com