Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Plantas Medicinales
versión On-line ISSN 1028-4796
Rev Cubana Plant Med vol.18 no.4 Ciudad de la Habana oct.-dic. 2013
ARTÍCULO ORIGINAL
In vitro determination of the antioxidant capacity of extracts and phenolic compounds from Ugni molinae Turcz. leaves
Determinación in vitro de la capacidad antioxidante de extractos y compuestos fenólicos de hojas de Ugni molinae Turcz.
PhD. Marcia Avello Lorca, PhD. Edgar Pastene Navarrete, MSc. Margarita González Riquelme, PhD. Magalis Bittner Berner, PhD. José Becerra Allende
University of Concepción. Concepción, Chile.
ABSTRACT
Introduction: Ugni molinae Turcz., (Myrtaceae) is a plant that is distributed in central-southern of Chile, including Juan Fernández Islands. The leaves have been used in folk medicine for diarrhea and oral infections due to their astringent and antiseptic properties.
Objective: antioxidant activity of two extracts (ethyl acetate and methanol) from U. molinae leaves was assessed in several assays.
Methods: HPLC (high performance liquid chromatography) for chemical analysis and in vitro antioxidant methods.
Results: HPLC profiling of both extracts showed the presence of flavan-3-ols (catechin) and gallic acid, flavonoids and caffeic acid derivates as main constituents. These extracts showed significant activity on 2,2-diphenyl-1-picrylhydrazyl (DPPH), cupric ion reducing antioxidant capacity (CUPRAC) and hydroxyl radical assays. It is noteworthy that bleaching of b-carotene-linoleate liposomes and copper-induced oxidation of human LDL were prevented by both extracts.
Conclusions: results suggest that polyphenol-rich extracts of U. molinae could slow down lipid peroxidation and limit free radical damage.
Key words: antioxidant capacity, lipid peroxidation, Ugni molinae.
RESUMEN
Introducción: Ugni molinae Turcz. (Myrtaceae) es una planta que se distribuye en el centro-sur de Chile, incluido el archipiélago de Juan Fernández. Las hojas se han utilizado en medicina popular para diarreas e infecciones bucales, por sus propiedades astringentes y antisépticas.
Objetivo: la actividad antioxidante de los dos extractos (acetato de etilo y metanólicol) de las hojas de Ugni molinae se evaluó mediante diferentes ensayos.
Métodos: HPLC (high performance liquid chromatography) para el análisis químico y los métodos antioxidantes in vitro.
Resultados: los perfiles HPLC de ambos extractos mostraron la presencia de flavan-3-oles (catequinas) y ácido gálico, flavonoides y derivados del ácido cafeico como constituyentes principales. Estos extractos mostraron actividad significativa frente a 2,2-difenil-1-picrilhidrazilo (DPPH), capacidad reductora del ión cobre (CUPRAC) y ensayos frente al radical hidroxilo. Es importante destacar que el blanqueamiento de liposomas de b-caroteno-linoleato y la oxidación producida por cobre de LDL (low density lipoprotein) humana fueron evitados por ambos extractos.
Conclusiones: los resultados sugieren que los extractos de Ugni molinae ricos en polifenoles podrían evitar la peroxidación lipídica y el daño de radicales libres.
Palabras clave: capacidad antioxidante, peroxidación lipídica, Ugni molinae.
INTRODUCTION
Various medicinal properties have been ascribed to natural herbs.1 Ugni molinae Turcz. (Myrtaceae), also known as «Murta», «Murtilla» or Chilean Guava, is a plant that grows in the south of Chile. Infusions of U. molinae leaves have long been used in traditional native herbal medicine to treat diarrhea and dysenteries.2 Studies of the chemical composition of the leaves indicate the presence of phenolic compounds like phenolic acids, flavonoids, triterpenes and tannins.3 These compounds can display antioxidant and anti-inflammatory properties with low toxicity.4 About 2 % of the oxygen used by normal cells is estimated to form reactive oxygen species (ROS).5 When ROS production overwhelms the numerous endogenous antioxidant defenses, a range of cellular structures and functions are damaged. This process, known as oxidative stress, leads to pathologies such as atherosclerosis, cancer and, ultimately, cell death.6 The main ROS are superoxide anion (O2.-) and hydroxyl radicals, which react with cell molecules such as lipids, proteins, carbohydrates, DNA, and lipoproteins.7 Regarding the latter, high plasma low density lipoprotein (LDL) levels are strongly linked to atherosclerosis, the major cause of coronary heart disease. Some studies have further demonstrated that atherosclerosis involves endothelial dysfunctions, monocyte infiltration, monophage activation, and smooth muscle cell proliferation.8,9 Moreover, oxidative modification of LDL plays a key role in triggering the molecular pathogenesis of atherosclerosis. Unlike intact LDL, which is metabolized through the receptor-mediated pathway, oxidized LDL (ox-LDL) appears in the circulation and tends to infiltrate the aortic endothelium.10 It is further oxidized in the intima until finally being taken up by macrophages. Thus, antioxidants able to inhibit LDL oxidation may reduce early atherogenesis and slow down the progression to more advanced stages.11,12 Antioxidants can limit the formation of free radicals in cells ameliorating their damage. The potential use of U. molinae extracts as antioxidants has been considered elsewhere.13 The molecular mechanisms underlying antioxidant effects of polyphenols have not yet been fully elucidated and are still a matter of considerable debate. However, it has been suggested that increased lipophilicity and subsequent cell partitioning result in restriction on membrane fluidity that could sterically hinder diffusion of free radicals, thereby decreasing the kinetics of free radical reactions.14 Thus, the antioxidant capacity of polyphenolic-rich beverages could be assessed using the rate of bleaching of stable free radicals, like 1,1-diphenyl-2-picryl-hidrazyl (DPPH) or 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid (ABTS), allowing us to evaluate the nonspecific free radical scavenging capacity.15 The aim of the present study was to investigate the antioxidant capacity of two U. molinae polyphenol-rich extracts using in vitro free-radical generating systems, lipid peroxidation assay, and protection of LDL against its oxidative modification induced by copper.
METHODS
Plant material
Ugni molinae leaves were collected from the areas surrounding Concepción, Chile, in April 2005, and were identified by Dr. Roberto Rodriguez of the Department of Botany, University of Concepción; a voucher specimen was deposited in the Herbarium under catalogue number CONC 146511. Leaves of U. molinae were washed, air-dried, and ground to a fine powder.
Extraction
Powdered U. molinae leaves (1.5 kg) were extracted in a Soxhlet apparatus successively with ethyl acetate (EAE) and methanol (ME). The extracts were filtered and concentrated under vacuum at 40 ºC, and dried under vacuum at room temperature. General phytochemical screening was conducted of both extracts to identify chemical entities with potential antioxidant activities, such as tannins and flavonoids.16,17
Total polyphenol content
Total polyphenol contents were spectrophotometrically determined according to Velioglu et al.18 using the Folin-Ciocalteu reagent (Sigma, MO, USA). Briefly, aliquots of test samples (0.5 mL) were mixed with 25 mL water, 2.5 mL Folin-Ciocalteu reagent, 10 mL 20 % Na2CO3, and completed to 50 mL with water, shaken for 30 min, and allowed to stand for 30 min. Absorbance of samples were registered at 765 nm using gallic acid as standard for calibration. Total polyphenol contents were expressed as mg of gallic acid equivalents per gram of extract.19
High performance liquid chromatography-ultraviolet (HPLC-UV) analysis
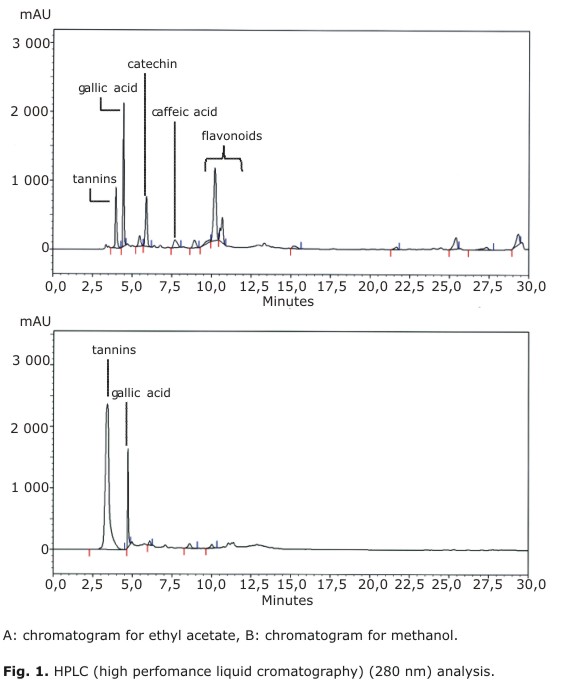
HPLC profiles of extracts were performed by HPLC according Motamed et al.20 using a system equipped with a LiChrospher 100 RP-18 column (5 mm, 125-4 mm). Elution was conducted under gradient with solvent A: 96 % ultrapure water, 3 % acetic acid, and 1 % acetonitrile, and B: 72 % ultrapure water, 3 % acetic acid and 25 % acetonitrile. The gradient started from 100 % A up to 100 % B (0-10 min), then 100 % B was maintained for 40 min, and finally the column was brought to initial conditions in 5 min (100 % A). Total running time was 55 min. Flow rate was 0.8 mL/min and detection was performed at 280 nm. The reagents used for analysis were all HPLC grade (Merck, Germany). Peak assignments were done by comparison of their tR with those of pure standard substances (gallic acid, catechin and caffeic acid), all purchased from Sigma.
2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
Antioxidant capacity was assessed using reduction of 1,1-diphenyl-2-picryl-hidrazyl (DPPH, Sigma , USA) according to Navarro et al.21 Aliquots of 750 mL of EAE and ME solutions (equivalent to 2- 200 mM GAE) were mixed with 1.5 mL of ethanol solution of DPPH (20 mg/mL). Absorbance was determined after 5 min at 517 nm in a spectrophotometer (Shimadzu UV-VIS 1601). Results were expressed as a percentage of DPPH reduction and Trolox Equivalent Antioxidant Capacity (TEAC) per gram of extract. Gallic acid and Trolox were used as standards.
Cupric ion reducing antioxidant capacity (CUPRAC) assay
CUPRAC indexes were determined according to Apak et al.22 For this purpose CuCl2 solution, 1.0 x 10-2 M, was prepared by dissolving 0.4262 g CuCl2 x 2H2O in water, and diluting to 250 mL. Ammonium acetate buffer 1.0 M at pH 7 was prepared by dissolving 19.27 g NH4Ac in water and diluting to 250 mL. Neocuproine (Nc) solution, 7.5 x 10-3 M, was prepared daily by dissolving 0.039 g Nc in 96 % ethanol, and diluting to 25 mL with ethanol. Trolox, 1.0 x 10-3 M, was prepared in 96 % ethanol. The assay was performed adding 1 mL 10-2 M Cu2+ + 1mL 7.5 x 10-3 M neocuproine + 1 mL 1 M NH4Ac + x mL 10-3 M antioxidant neutral solution + (1.1- x) H2O; total volume = 4.1 mL; measure final absorbance at 450 nm.
Hydroxyl Radical (OH·) Scavenging Assay
Scavenging of the hydroxyl radical (OH·) was measured by the deoxyribose method.23,24 Hydroxyl radicals were generated by reacting 50 mL of Fe+3 (20 mM) with 50 mL of H2O2 (42 mM) and 10 mL of ascorbate (50 mM). Then, 10 mL of either EAE or ME extracts (equivalent to 2 - 200 mM GAE) and 100 mL of deoxyribose (2.8 mM) in PBS buffer (10 mM, pH 7.4) were added. The corresponding mixtures were incubated at 37 ºC for 1 h. Then 1 mL of trichloroacetic acid (2.8 %) and 1 mL of thiobarbituric acid (TBA) were added and heated at 100 ºC for 15 min. After cooling, absorbance of reddish adducts was measured at 532 nm using a spectrophotometer (Shimadzu UV-VIS 1601). Results were expressed as a percentage of OH· inhibition. Gallic acid was used as the standard.
b-Carotene-Linoleate Bleaching Assay
The effect of U. molinae extracts on the oxidation of b-carotene and linoleic acid charged liposomes was determined according to the method of Velioglu et al.18 with slight modifications. 0.5 mL of b-carotene (Sigma, USA) (2 mg/mL) in chloroform was added to a 10 mL aliquot of linoleic acid (Sigma, USA) and 100 mL of the tensoactive agent Tween 80; afterwards EAE and ME fractions were added. After evaporation and drying under vacuum at room temperature, 25 mL of oxygenated water were added and shaken for 1 min. Liposomes were then subjected to thermal oxidation at 50 ºC in a thermoregulated cuvette in a spectrophotometer (Shimadzu UV-VIS 1601) for 70 min. Absorbance was recorded at 470 nm every 5 min. Results were expressed as a percentage of b-carotene and linoleic acid protection. Gallic acid was used as a standard.
Protective Effects on LDL Oxidation by Cu2+
Blood samples were collected from young healthy donors after fasting overnight for 12 hours. EDTA (5 mM) was used as an anticoagulant. Blood was centrifuged at 800 x g at 4 ºC for 15 min. Human LDL (d=1.019-1.063 g/mL) was prepared from the separated plasma by sequential flotation ultracentrifugation at 4 ºC as described by Schumaker and Puppione.25 Prior to the oxidation experiences, LDL was dialyzed overnight against PBS. Aliquots of LDL (6.82 mg/mL) were incubated with Cu+2 (7 mM) at 37 ºC to induce lipid peroxidation according to Gugliucci.26 LDL was pre-incubated with the extracts for 10 min before adding Cu2+. Incubation was carried out at 37 ºC for 24 h. At this time, oxidation was stopped by cooling on ice and by the addition of butylated hydroxytoluene (BHT, 40 mM), a radical-trapping antioxidant, and EDTA (1 mM). Rutin was used as a control. Formation of diene conjugates was determined by UV absorption at 234 nm,27 using a spectrophotometer (Shimadzu UV-VIS 1601). Fluorescence was measured at an excitation wavelength of 360 nm and an emission of 410 nm, as previously described by Steinbrecher,28 using a spectrofluorophotometer (Shimadzu RF-5301 PC). Electrophoretic mobility assay was determined on 1 % agarose gels in barbital buffer (pH 8.6) and by staining with Coomassie Brilliant Blue (Sigma B0149, USA), according to Noble.29 Electrophoresis was carried out in a horizontal chamber (30 mA, 2 hours) (Merck, Darmstadt, Germany).
Statistical Analysis
Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Dunnett's t-test for multiple comparisons. Differences were considered significant at p< 0.05.
RESULTS
Total Polyphenol Content and HPLC-UV Analysis
Total polyphenolic contents (TPC, Table 1) in EAE and ME were 87 and 279 mg GAE/g, respectively. However, as shown in Figures 1A and 1B both extracts displayed a different HPLC profile. In EAE, it was possible to identify catechin, caffeic acid and traces of free gallic acid as in previous studies.4 In fact, gallic acid was concentrated in ME along with other polyphenols, which presumably could be a complex moiety of gallotannins. In our HPLC system, these compounds elute as an unresolved peak around tR= 3.4 min. As expected, flavan-3-ols, and some flavonoid glycosides were concentrated in EAE. The latter compounds elute between tR= 9.0-12.0 min and present a second absorption band at 350 nm (data not shown). Neither procyanidins nor unbound flavonoids were detected in the extracts. To complement the chemical information, Aguirre and colleagues have recently identified assiatic, corosolic, oleanolic, betulinic and ursolic acids in U. molinae leaf extracts.3,30
DPPH Assay
The antioxidant capacity of EAE and ME prepared from U. molinae was evaluated using different assays. Both extracts were able to decolorize the stable DPPH probe. As shown in Table 2, U. molinae extracts stabilized DPPH in a concentration-dependent manner. Thus, 100 % DPPH stabilization was reached with 200 µM GAE of EAE and ME. For gallic acid (pure standard), 100 % scavenging activity was reached with a concentration of 600 µM, suggesting that U. molinae possesses other antioxidant molecules acting synergistically. As shown in Table 1, TEAC-DPPH values for EAE and ME were 990 and 2447 µmol Trolox equivalents per gram, respectively.
CUPRAC Assay
Values were similar to those obtained by CUPRAC assay (972 and 2880 for EAE and ME, respectively). These TEAC indexes indicate that ME possesses almost 2.5-3 times more antioxidant activity than EAE extract. The same relationship could be observed for total polyphenolic contents (3.2 times), which suggests that these compounds would explain a great part of the antioxidant potential of both extracts. In the case of EAE, polyphenols represent 8.7 % of the total weight, while in ME this value was 27.9 %.
Hydroxyl Radical (OH·) Scavenging Assay
Scavenging of hydroxyl radical was concentration-dependent also (Table 2). The same concentration of extracts (200 µM GAE) inhibited the hydroxyl radical formation at 83.3 %, while gallic acid it does up to 60 %.
b-Carotene-Linoleate Bleaching Assay
Results suggest that these extracts would protect polyunsaturated fatty acids (PUFAs), which lose hydrogen atoms in the initial phase of peroxidation.31 In order to evaluate those lipid peroxidation preventive properties, we used the ß-carotene linoleate bleaching assay. The results of this assay are shown in Table 2, where it is clearly seen that U. molinae extract effectively protected the ß-carotene linoleate charged liposomes against O2/temperature induced oxidation. At equal concentrations, both EAE and ME effectively protected liposomes from oxidative damage up to 85.1 % and 91.9 %, respectively. Protection of ß-carotene linoleate charged liposomes was observed with gallic acid concentrations over 600 µM (95.4 %).
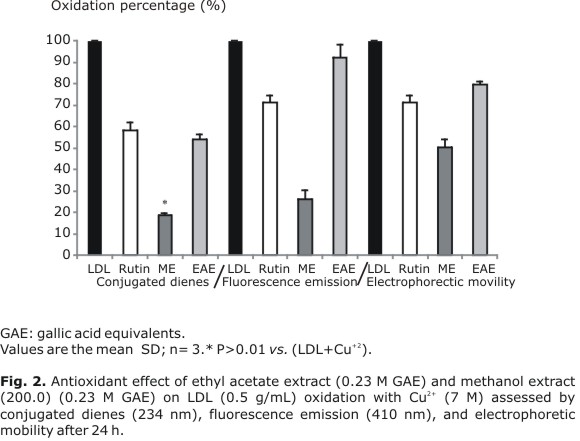
Protective Effects on LDL Oxidation by Cu2+
In the present study, copper-induced LDL oxidation was inhibited by U. molinae extracts. As shown in Figure 2, this protection was assessed through conjugated dienes, fluorescence emission, and electrophoretic mobility assays. In our experimental conditions, ME (0.23 µM GAE) was more effective against copper-induced LDL oxidation than EAE (0.23 µM GAE). Compared with the untreated control (copper + LDL), the levels of conjugated dienes at 24 hours were decreased about 83 % with ME. When EAE was used, only 52 % LDL protection was observed. Fluorescence emission at 24 hours decreased (75 %) with ME. Again, EAE attained a poor level of LDL protection (7 %).
As shown in Figure 2, when LDL was incubated with ME, its electrophoretic mobility at 24 hours was 50 % of the oxidized LDL mobility. On the other hand, the observed LDL protection with EAE was 18 % whereas for rutin (flavonol glycoside) it ranged between 30 and 40 %.
DISCUSSION
The above-mentioned results could evidence the hydrogen/electron donating properties associated to the polyphenols contained in U. molinae extracts (DPPH, CUPRAC and hydroxyl radical assays).
The oxidation process observed in the ß-carotene linoleate system could be summarized in three steps: hydrogen loss, molecular reordering, and a chain reaction that generates lipid peroxidation by severing bonds in the lipophilic medium. The fact that polar antioxidants such as those found in U. molinae are more effective in lipidic systems was previously observed for other plant sources.32 Such a phenomenon is based on the protective effect exerted by the air-lipidic-water interface created between the lipid system and the hydrophilic extract. In this interface, where lipid oxidation is initiated, polyphenols exert their effects against air-contact oxidation. In contrast, when this protection is absent, oxidation processes could continue leading to lipoperoxidation.33,34
Tissue injury observed in certain pathologies (atherosclerosis, cancer, liver disease, and the aging process) has been associated to oxidative stress. The reactive oxygen intermediaries generated during this process could modify lipids and proteins. In fact, it is thought that oxidative LDL modification plays a central role in the pathogenesis of atherosclerosis and coronary heart disease.35 Therefore, limiting LDL modification through the intake of natural antioxidants from foods and other vegetal sources could prevent such complications. LDL oxidation could lead to a reduction of the LDL surface charge density. Oxidized cholesterol esters and triglycerides are more polar and amphipathic than their precursors; therefore, it would be energetically less stable for them to remain in the hydrophobic region of LDL. Migration of these oxidized molecules from the hydrophobic region near the surface of LDL, reduces particle surface charge density and thus the surface electric potential of lipoproteins, leading to their aggregation. Regarding the latter, PUFA molecules may play a leading role in such surface charge density reduction. Thus, the aldehydes, derived from breakage of the nonpolar side of the fatty acid ethyl bond, migrate to the surface of the LDL because of the polarity of the aldehyde group and its ability to form hydrogen bonds. Hydroxyl -or peroxyl- fatty acids reposition themselves so that both the hydroxyl or peroxyl group and the carboxyl group reside in the polar and charged head-group region.36,37 Arrival of these aldehyde, hydroxyl, and peroxyl group at the head-group region would occupy more space without additional charges and thus reduce charge density.
LDL results revealed that ME and EAE extracts decreased diene formation through the stabilization of double bonds that inhibit molecular reordering. Fluorescence emission results indicated that unsaturated lipid oxidation end-products were not formed and LDL proteins remained unchanged. These results differ significantly from those observed for the untreated control (copper + LDL). The electrophoretic mobility assay allows to register certain changes in the electric charges of the lipoprotein because lipoprotein oxidation is manifested as changes in electrical charges and, consequently, higher electrophoretic mobility. Therefore, our results suggest that U. molinae extracts limit these changes by slowing LDL oxidation. It must be pointed out that the results of this last assay could be in part associated with the chelating ability of gallotannins and not necessarily with direct scavenging properties upon radicals generated within the LDL particle. Thus, as radicals go deeper into the interior of the LDL particle, the efficacy of hydrophilic polyphenols becomes smaller. U. molinae has ursolic, oleanolic, betulinic, assiatic and corosolic acids.3,30 Antioxidant properties of these pentacyclic triterpene acids have been reported elsewhere, and their presence in U. molinae extracts could explain in part the protection of LDL by scavenge radicals located near the hydrophobic core of this lipoparticle.38 The chemistry of LDL oxidation has been studied extensively. Many small molecules inside LDL, including those in the hydrophobic core, are vulnerable to oxidants. The following oxidation reactions have been observed: ß-carotene to retinol and retinoic acid; alpha-tocopherol to? alpha-tocopherol hydroquinone; PUFA to hydroxy- and peroxy-fatty acids and other derivatives, including malondialdehyde and other short-chain aldehydes; ubiquinol to ubiquinone; and cholesterol to cholesterol oxides. The aldehydes formed could react with amine in apoB and lead to cross-linking of lysine residues in different regions of apoB. This type of lipid/protein conjugation has been detected for 4-hydroxy-2-nonenal-modified histidine, lysine, and cysteine residues by amino acid analysis of oxidized LDL.39 All the modifications affect the integrity of the LDL structure and lead to a change in the LDL surface electric property that is often the cause of protein aggregation.
The results observed for EAE and ME extracts could be originated in their different phytochemical composition. The concentrations of the extracts used in the assays were chosen in terms of the sensitivity of each method. Therefore, these results are just an in vitro approximation to assess the potential antioxidant activity of this phytocomplex. A large part of the U. molinae gallotannins could potentially release gallic acid in the gastrointestinal ambient. Regarding the latter, it is clear that the intent to predict the antioxidant activities of such compounds using in vitro data, requires more studies. For instance, the bio-accessibility of hydrolizable polyphenols has been evaluated using in vitro simulated gastrointestinal models.40 The authors investigated the in vitro bio-accessibility of polyphenols present in the Spanish Mediterranean diet. They estimated that about 48 % of dietary polyphenols are bioaccessible in the small intestine, while 42 % become bioaccessible in the large intestine. Previously, we observed that acute administration of U. molinae infusions to seven healthy volunteers produced positive changes in their plasma antioxidant capacity (ORAC) after three days of oral administration.
In our research, two U. molinae extract fractions were separately evaluated in order to dissect the underlying the protective effects against lipid peroxidation and free radical damage. Both extracts are rich in polyphenols, though different in their chemical nature. Nevertheless, at least in part, such composition underlies the benefits described in folk medicine. In vivo studies are currently underway to verify whether these properties are homogeneous in U. molinae samples extracted from different geographic locations.
REFERENCES
1. Ivanova D, Gerova D, Chervenkov T, Yankova T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J Ethnopharmacol. 2005;96:145-50.
2. Hoffmann A. Flora silvestre de Chile, zona Araucana. Chile: Fundación Claudio Gay; 1991. p. 160.
3. Aguirre MC, Delporte C, Backhouse N, Erazo S, Letelier ME, Cassels BK, et al. Topical anti-inflammatory activity of 2 alpha-hydroxy pentacyclic triterpene acids from the leaves of Ugni molinae. Bioorganic Medicinal Chemistry. 2006;14:5673-7.
4. Rubilar M, Pinelo M, Ihl M, Scheuermann E, Sineiro J, Nuñez MJ. Murta leaves (Ugni molinae Turcz.) as a source of antioxidant polyphenols. J Agricultural Food Chemistry. 2006;54:59-64.
5. Devany S, Adams-Huet B, Jialal I. Comparison of rr-alpha-tocopherol and all-racemic alpha-tocopherol on LDL oxidation. Arteriosclerosis, Thrombosis, Vascular Biology. 1997;17:2273-9.
6. Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organism. Physiological Reviews. 1979;59:527-605.
7. McCall MR, Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radical Research Communications. 1999;26:1034-53.
8. Sohal RS, Weindruch R. Oxidative stress, caloric restriction and aging. Science. 1996;273:59-63.
9. Steinbrecher UP. Beyond cholesterol: modification of low density lipoprotein that increases its atherogenicity. J Biological Chemistry. 1987;262:3603-10.
10. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801-9.
11. Palinski W, Rosenfeld ME, Yla-Hertuala S, Gurther GC, Socher SA, Butler SW, et al. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:1372-6.
12. Parthasarathy S, Satinberg D, Witztum JL. The role of oxidized low density lipoproteins in the pathogenesis of atherosclerosis. Annual Review Medicine. 1992;43:219-25.
13. Avello M, Pastene E. Actividad antioxidante de infusos de Ugni molinae Turcz. (Murtilla). Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2005;4:33-9.
14. Arora A, Nair MG, Strasbur M. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radical Research Communications. 1998;24:1355-60.
15. Ellnain-Wojtaszek M, Kruczynski Z, Kasprzak J. Investigation of the free radical scavenging activity of Ginkgo biloba L. leaves. Fitoterapia. 2003;74:1-6.
16. Domínguez X. Métodos de investigación fitoquímica. México: Limusa; 1973. p. 81-149.
17. Shinoda J. A new biologically active flavone glycoside from the roots of Cassia fistula Linn. J Pharmaceutical Society Japan. 1928;48:214.
18. Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agricultural Food Chemistry. 1998;46:4113-7.
19. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American J Enology Viticultur. 1965;16:144-58.
20. Motamed B, Bohm JL, Hennequin D, Texier H, Mosrati R, Barillier D. Development of an HPLC method for the determination of phenolic by-products: optimization of the separation by means of the experimental designs methodology. European J Analytical Chemistry. 2000;28:592-9.
21. Navarro MP, Montilla A, Marín J, Jiménez MP. Free radical scavenger and antihepatotoxic activity of Rosmarinus tomentosus. Planta Medica. 1993;59:312-3.
22. Apak R, Güclü K, Demirata BM, Özyürek SE, Celik B, Bektasoglu K, et al. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496-547.
23. Halliwell B, Gutteridge J, Aruoma OI. The deoxyribose method: a simple «test-tube» assay for determination of rate constants for reactions of hydroxyl radicals. Analytical Biochemistry. 1987;165:215-9.
24. Winterbourn CC, Sutton H. Iron and xanthine oxidase catalyze formation of an oxidant species distinguishable from OH: comparison with the Haber-Weiss reaction. Biochemical Biophysical Research Communications. 1986;244:227-341.
25. Schumaker VN, Puppione D. Sequential flotation ultracentrifugation. Methods Enzymology. 1986;128:155-70.
26. Gugliucci A. Antioxidant effects of Ilex paraguariensis: induction of decreased oxidability of human LDL in vivo. Biochemical Biophysical Research Communications. 1996;224:338-44.
27. Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radical Biology Medicine. 1989;6:67-75.
28. Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modification of low density lipoproteins that increase its atherogenicity. New England J Medicine. 1989;320:915-24.
29. Noble RP. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Research. 1968;9:693-700.
30. Delporte C, Backhouse N, Inostroza V, Aguirre MC, Peredo N, Silva X, et al. Analgesic activity of Ugni molinae (murtilla) in mice models of acute pain. J Ethnopharmacol. 2007;112:162-5.
31. Belinky PA, Aviram M, Fuhrman B, Rosenblat M, Vaya J. The antioxidative effects of the isoflavan glabridin on endogenous constituents of LDL during its oxidation. Atherosclerosis. 1998;137:49-61.
32. Khan MA, Shahidi F. Oxidative stability of stripped and nonstripped borage and evening primrose oils and their emulsions in water. J American Oil Chemists' Society. 2000;77:963-8.
33. Frankel E. Food antioxidants and phytochemicals: Present and future perspectives. European J Lipid Science Technology. 1999;101:450-5.
34. Porter WL. Paradoxical behavior of antioxidants in food and biological systems. Toxicology Industrial Health. 1993;9:93-122.
35. Cox DA, Cohen ML. Effects of oxidized low-density lipoprotein on vascular contraction and relaxation: clinical and pharmacological implications in atherosclerosis. Pharmacological Reviews. 1996;48:3-19.
36. Kellner BM, Cadenhead DA. Monolayer studies of hydroxyhexadecanoic acids. J Colloid Interface Science. 1977;63:452-60.
37. Kellner BM, Cadenhead DA. Monolayer studies of methylhydroydecanoates. Chemistry Physics Lipids. 1978;23:41-8.
38. Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radical Biology Medicine. 2010;49:503-15.
39. Uchida K, Toyokuni S, Nishikawa K, Kawakishi S, Oda H, Hiai H, et al. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry. 1994;33:12487-94.
40. Saura-Calixto F, Serrano J, Goñi I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chemistry. 2007;101:492-501.
Recibido: 14 de marzo de 2013.
Aprobado: 30 de mayo de 2013.
Marcia Avello Lorca. Faculty of Pharmacy, University of Concepción. Casilla 237, Concepción, Chile. Fax: (56-41) 2207086. Correo electrónico: maavello@udec.cl