Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Plantas Medicinales
versión On-line ISSN 1028-4796
Rev Cubana Plant Med vol.20 no.3 Ciudad de la Habana jul.-set. 2015
ARTÍCULO ORIGINAL
Effects of ethanol extract of Cenostigma macrophyllum Tul. (caneleiro) on reproductive parameters of female rats
Efectos del extracto etanólico de Cenostigma macrophyllum Tul. (caneleiro) sobre parámetros reproductivos de ratas hembras
MSc. Denise Barbosa Santos, MSc. Jamylla Mirck Guerra de Oliveira, MSc. Martins Neto Bueno, MSc. Paulo Alex Bezerra Sales, Dr. Charllyton Luis Sena Costa, Dra. Mariana Helena Chaves, Dra. Maria do Carmo Carvalho e Martins, Dr. Amilton Paulo Raposo Costa
Universidade Federal do Piauí. Brasil.
ABSTRACT
Introduction: Cenostigma macrophyllum Tul., known as Caneleiro presents anti-inflammatory, antinociceptive, antiulcerogenic activities.
Objective: to evaluate the effects of the ethanol extract from C. macrophyllum Tul. leaves on the reproductive parameters of female Wistar rats.
Methods: for estrogenic activity assay were used 32 ovariectomized female rats (n= 8) treated for four days: I- NaCl 0.9 %, 10 mL/kg of body weight orally + corn oil 1 mL/kg intramuscular (IM); II- NaCl 0.9 % orally + estradiol 10 µg/kg bw IM; III- extract 500 mg/kg orally + corn oil IM and IV- extract orally + estradiol IM. The uteri were weighted. The estrous cycle evaluation was made using 12 female rats examined daily by vaginal smear, before, after and during the treatment (16 days) with extract (500 mg/kg). The each estrous cycle phase duration and the interval between the cycles were measured. For reproductive toxicity study were used 16 female rats (n= 8) that were treated orally during all gestational period with: NaCl 0.9 % 10 mL/kg and extract 500 mg/kg. The rats were anesthetized and laparotomized for uterus and fetus evaluation. Furthermore, the heart, liver and kidneys were collected and submitted to histopathological evaluation.
Results: the uterine weight did not differ between the groups. There was a decrease in the estrous number and an increase in the estrous cycle duration in the treatment and pos treatment groups. The extract did not cause toxicity in pregnant rats nor signs of alterations in the newborns. The histopathological analysis did not reveal significant alterations in the organs.
Conclusion: the extract interferes in the estrous cycle but does not present toxicity over gestation and nor alters macroscopically or microscopically the analyzed organs structures.
Key words: reproductive toxicity, estrogenic activity, estrous cycle.
RESUMEN
Introducción: Cenostigma macrophyllum Tul. conocido como Caneleiro demuestra actividades antiinflamatorio, antiulcerogénico y antinociceptiva.
Objetivo: evaluar los efectos del extracto etanólico de hojas de C. macrophyllum Tul. en los parámetros de reproducción de ratas Wistar.
Métodos: para el ensayo de actividad estrogénica se utilizaron 32 ratas hembras ovariectomizadas (n = 8) tratadas durante cuatro días: I- NaCl 0,9 % 10 mL/kg de peso corporal por vía oral + aceite de maíz 1 mL/kg por vía intramuscular (IM); II- NaCl 0,9 % por vía oral + estradiol 10 µg/kg IM; III - extracto 500 mg/kg por vía oral + aceite de maíz IM y IV- extracto oral + estradiol IM. Los úteros fueron pesados. Para la evaluación del ciclo estral, 12 ratas hembra fueron examinadas diarias por frotis vaginal, antes, después y durante el tratamiento (16 días) con el extracto (500 mg/kg). Se midió la duración de cada fase del ciclo estral y el intervalo entre ciclos. Para el estudio de toxicidad reproductiva se utilizaron 16 ratas hembras (n = 8) que fueron tratados, por vía oral, durante todo el período de gestación con NaCl 0,9 % (10 mL/kg) y extracto (500 mg/kg). Las ratas fueron anestesiadas y laparotomizadas para evaluar el útero y los fetos. Además, se recogieron el corazón, el hígado y los riñones y se sometieron a evaluación histopatológica.
Resultados: el peso del útero no fue diferente entre los grupos. Hubo una disminución en el número de estro y un aumento en la duración del ciclo estral en los grupos tratamiento y post-tratamiento. El extracto no causó toxicidad en ratas preñadas y ni muerte fetal o signos de alteraciones en los recién nacidos.
Conclusiones: el extracto interfiere en el ciclo estral, pero no presenta toxicidad sobre la gestación y ni altera macroscópicamente o microscópicamente las estructuras de los órganos analizados.
Palabras clave: toxicidad para la reproducción, actividad estrogénica, ciclo estral.
INTRODUCTION
The importance of researches with medicinal plants in developing countries is evident, especially for Brazil, in which the growth of research in this area is below 10 % per year, even though the country has the bigger plant biodiversity in the world. This reality difficults the rational use of medicinal plants and the production of phytotherapics due to lack of information about the effectiveness and safety of these products, stimulating the increasing exclusion of native species from medicine and from official pharmaceutical guides.1-5
Many plants utilized as medication or for human and animal nutrition are rich in flavonoids, some of them with estrogenic activity. Many beneficial effects of soy (Glycine max) flavonoids were demonstrated like the prevention of many human chronic illnesses such as osteoporosis, menopause alterations, decrease in the incidence of prostate, colon and breast cancer, hypercholesterolemia, atherosclerosis and heart diseases.6-8
The estrogenic and antiestrogenic activity of phytoestrogens depends on the concentration of the endogen sexual steroids and the specific target organ. This effects variation can be explained by the existence of fetal death and of two types of estrogen receptors (ER): α and β. The α-receptors (ER-α) are the main receptors found in the breast and uterus, and the β-receptors (ER-β) are the ones that predominate in the bones and in the cardiovascular system.9
Besides the effects via estrogen receptors, many of the possible effects of isoflavones and other phytoestrogen can be attributed to the metabolic activity that involves other systems not associated with these receptors, including the influence over enzymes such as ATPase, inhibition of DNA, topoisomerase, antioxidants effects over lipids, lipoproteins and DNA, besides effects on the transport of glucose and of ions. Effects of the phytoestrogens over the proteic synthesis, cell proliferation, angiogenesis, growth factors, smooth vascular muscle and cellular differentiation have also been related. 10,11
The family Leguminosae Adms, of which the Caneleiro is part of (Cenostigma. macrophyllum Tul.), is constituted by species in which preliminary phytochemical study of extracts and fractions demonstrated the presence of substances of the class of the flavonoids,12-14 specifically biflavonoids.15
Different evidences point to the capacity that the biflavones have of increasing the degree of lipolysis in adipocytes. Such characteristics associated to the anti-inflamatory activities and vascular relaxants turn these substances in potential therapeutical agents for the regressive amyotrophic panicle disease or liosclerosis, commonly known as cellulites, which is characterized by presenting venous stasis and/or venous chronic insufficiency. The reduction of the sclerotic phenomenon can be obtained with the topic application of substances that moderate the microcirculation of the skin and that have lipolytic activity.16-17
The Cenostigma macrophyllum Tul. variety acuminata Teles Freire, occurs in the West-Central, Southeast and Northeast regions, where it is observed in the states of Maranhão, Piauí, Ceará, Pernambuco and Bahia.18 It has an ample distribution in the city of Teresina-PI, especially in the afforestation of streets and squares, for this reason it was chosen as the tree symbol of the city.
Many plants of the Leguminosae family, among them the Caneleiro, are popularly used as an antidiarrheal, laxatives, gum’s astringents and in the treatment of wounds. In raw extracts of Cenostigma it was observed antiinflamatory activity, antinociceptive, antiulcerogenic, hepatoprotective, antioxidant, hypoglycemic, antimicrobial, inhibitory activity of Walker tumor in rats, besides the restriction of the coronary flow and secondary electrocardiographic changes.3,19-21
The evaluation of estrogenic and antiestrogenic activity can be done by researching the effects of the suspected material on the uterus, which is a very sensitive organ to estrogen, increasing the volume and accumulating liquid in its interior when exposed to little doses of estrogen.22 Since the estrogens derived from plants and other sources have, besides specific therapeutical potential, also potential to change the estrous cycle, the evaluation of the estrogenic activity of plants with therapeutical potential is important. For this purpose, an important method is the determination of the phases of the estrous cycle in female rats, because of the short duration of the cycle in this specie.23
The present research has the goal of investigating a possible toxicity of the ethanol extract from the leaves of Cenostigma. macrophyllum Tul. on reproductive and also histopathological parameters in female rats.
METHODS
Vegetal material
Fresh leaves of the specie Cenostigma macrophyllum Tul var. acuminata Teles Freire were collected from the headquarters of the Workers’ Union of the Federal University of Piauí (SINTUFPI), Teresina-PI, in 24 of July of 2006, being identified by Prof. Maurício Teles Freire from the Department of Biology at UFPI whom exsicata n° TEPB 10.374 is located in the Herbarium Graziela Barroso-UFPI.
The extract was obtained from the leaves of C. macrophyllum that were air-dried (45±1 °C), crushed (knife mill Marconi, São Paulo, Brazil) and submitted to maceration process five times with ethanol at 70 % (Vetec, Rio de Janeiro, Brazil) at room temperature. After removal of the solvent on the rotary evaporator (Quimis 344B2, Sao Paulo, Brazil) at 55 °C under reduced pressure, the ethanol extract was maintained under refrigeration, at 4 ºC, until its use.
Animals
Wistar female rats were used weighting 180-250 g, raised and kept in the vivarium from the Agrarian Science Center-UFPI, in regimen of 12 h with light and 12 h in darkness, in a room with a refrigeration system with air exits and with free access to water and food (FRI-LAB Rats - Fri-Ribe).
Ovarietcomy of the rats
The female rats were anesthetized with ketamine + xylazine and were laid down on their sides. In each side, in the region of the flank, an incision of 1.0 to 1.5 cm was made on the skin and in the subcutaneous tissue. Next, the peritoneal cavity was open by divulsion of the muscles and peritoneum layers. Through each incision, each corresponding ovary was located. A ligature was then made in the uterus-tube junction involving all of the vascularization of the ovary and a section of the tube and other structures between the ligature and the ovary were removed. The uterine horn was replaced in the abdominal cavity and the incision was sutured. The animals were kept under rest for a period of 20 days so that they could recover from the surgical trauma and for the uterine involution to happen.
Estrogenic activity
After 20 days of the spaying the female rats were distributed, randomly, in four groups (n = 8) and received the following treatments:
- Group I: NaCl 0.9 %, 10 mL/kg of body weight (bw) orally + corn oil 1 mL/kg bw intramuscular (IM).
- Group II: NaCl 0.9 %, 10 mL/kg of body weight (bw) orally + estradiol 10 µg/kg bw) IM.
- Group III: extract 500 mg/kg bw orally + corn oil 1 mL/kg bw IM and group IV- extracto 500 mg/kg bw orally + estradiol 10 µg/kg bw IM.
The treatment was performed during 4 days and in the fifth day, the rats from all groups were euthanized by excess of anesthetic and soon after the removal, cleaning and weighting of the uterus were done. The weights obtained were converted to 100 g of body weight and were submitted to a statistical analysis.
The protocols used in the present research were according to the current ethical and technical guidelines for the use of animals in experiments, having been approved by the Ethics in Research Committee of the Federal University of Piauí created by the Resolution CNS 196/96.
Reproductive toxicity
Sixteen adult female rats, which were divided, randomly, in two groups (n=8): Control and Extract were used. The rats were examined daily as to which phase of the estrous cycle they were, using a fresh vaginal scrub. Those that were detected in proestrus were mated with a fertile male and the presence of spermatozoids in the scrub in the morning following the mating process was taken as an indicative sign of the pregnancy (first day). Once they were pregnant, the rats received two treatments related below, from the first to the twentieth day of pregnancy, orally:
- Control: they were treated with 10 mL/kg of body weight, of NaCl 0.9 %.
- Extract : they received extract 500 mg/kg in the volume of 10 mL/kg of body weight.
Euthanasia, exam and weighting of the animals
After the period of treatment, in the twentieth first day of pregnancy, the rats were anesthetized and submitted to caesarian section, for the removal and the evaluation of the pregnant uterus and its contents. The number of live fetus was counted, as well as the number of implantation sites. Then, the individual weighting of the fetus and of the placenta was done. The fetus was examined macroscopically and evaluated according to the presence of anomalies and/or congenital malformations.
Systemic toxicity
The systemic toxicity was evaluated by the analysis of histological cuts of the heart, kidneys and liver. After the procedures of evaluation of the reproductive toxicity, the removal of the cited organs was then made from the mother rats. Tissues’ sections were fixed in buffered formalin (formaldehyde solution at 10 %), after 24 h, were resectioned and submitted to the histopathological processing: dehydrating with a crescent series of alcohol (70 to 100 %), diaphanisation in xilol, saturation and inclusion in paraffin, according to the usual methods. In a microtome, the tissues’ fragments were sectioned at a thickness of 3.0 µM and subsequently submitted to the hematoxylin-eosin staining protocol and examined with a light microscope.
Evaluation of the Estrous cycle
Were used 12 female Wistar rats, weighting between 180-220g. All of the rats were examined daily, between 8:00 and 9:00 am, during 48 days concerning the phase of the estrous cycle, being 16 days before, 16 days during and 16 days after the treatment with the extract under study.
The vaginal smears were collected with a plastic pipette containing approximately 10 µL of saline (NaCl at 0.9 %) and deposited on a glass microscope slide and analyzed using a light microscope, with the objectives of 10 and 40x. The treatment consisted in the application of the extract from leaves of Caneleiro (Cenostigma. macrophyllum Tul.), orally, in the dose of 500 mg/kg of body weight, daily, during 16 days. After the end of the treatment, the female rats were still evaluated during 16 additional days and the duration of each phase of the cycle was used as a parameter for the analysis.
Statistical analysis
The results of the estrogenic activity were submitted to the One-Way Test (ANOVA), followed by the test of Student Newman-Keuls for the comparison of the means. The reproductive toxicity data was compared by the t Student test and the results of the evaluation of the estrous cycle using the Dunnett’s test. All of the analysis was done using a statistical software Sigma Stat. The level of significance used was of 5 % (p < 0.05).
RESULTS
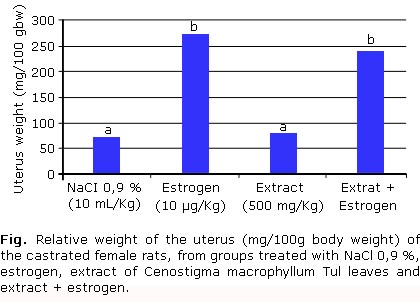
Was no observed difference in the uterus weight from rats treated with the extract when compared to the NaCl 0.9 % group, which demonstrates the absence of estrogenic activity in the ethanol extract of Cenostigma macrophyllum Tul. (Fig.). When was compared uterine weight of rats treated with estrogen relative to the group that received estrogen and extract, no significant difference was detected. This result indicates absence of anti-estrogenic activity in the extract.
The mean of reproductive parameters evaluated in pregnant rats showed no significant difference between treatments (table 1). Also no morphological or functional changes in mothers or fetuses were observed, showing that the ethanol extract of Cenostigma macrophyllum Tul. has no toxicity over pregnancy of rats.
There was a decrease in the duration of the estrus stage during and after treatment with the ethanol extract of Cenostigma macrophyllum Tul., whereas the overall duration of the estrous cycle was longer during these periods (table 2).
Histopathological analysis revealed no signs of degenerative, inflammatory or necrotic changes in the organs of rats subjected to treatment with extract when compared to the control group (data not shown).
DISCUSSION
Chemical or physical agents can affect the female reproductive system in any period of the cycle of their life. This system starts to be formed in the beginning of pregnancy, but the structural and functional maturation only is completed at the start of puberty.24 In the prenatal and postnatal phases, the sexual organs and the central nervous system, which still are not differentiated, can be affected also by substances present in the maternal blood by means of the placenta. Moreover, during the critical periods of development the individuals are more vulnerable to the action of chemical substances in function of the lesser metabolic and excretory capacity and the absence of many feedback mechanisms of the endocrine system.25
The evaluation of the toxic effects of a drug includes researches about possible results on the maternal organism, because the maternal toxicity, that is defined as a transitory or permanent alteration in the maternal physiology with potential to cause adverse effects in the offspring during the embryo development or postnatal, is intimately associated with particular malformations of each species.26
One of the effects researched in plants with therapeutical potential is the estrogenic and antiestrogen activity. The figure presents the means of the uterine weights of the castrated female rats submitted to several treatments. It is observed that there was no difference regarding the weight of the uterus of rats treated with extract when compared with the group treated with NaCl 0.9 %. This demonstrates the absence of the estrogenic activity in the ethanol extract of C. macrophyllum Tul. On the other hand, in the comparison between the groups treated with estrogen and extracts associated with estrogen, a significant difference was not observed, revealing the lack of antiestrogenic activity of the extract under study. Evaluating the quality of the experiment carried out, it was verified that the uterine weight of the female rats treated with estrogen was significantly superior to the ones treated with NaCl 0.9 % (p < 0.05), indicating that the controls, negative and positive, were well established and that the hormone used has satisfactory activity.
The implantation is the process by which the embryo does physical contact and intimate physiological contact with the maternal endometrium for the establishment of the gestation. Although there is a variation in the process between species, certain basic events are similar. The fundamental characteristic of this process is the synchronized development of the embryo for the stage of blastocyst and the differentiation of the uterus for the receptive condition. Following, interaction happens between the blastocyst activated and the uterine epithelium to start the implantation.27
The implantation occurs normally in the 4th or 5th day in rodents, interferences during this period can lead to losses in the embryonic implantation. 28,29 The ingestion of the extract did not cause modifications in the number of implantation sites (table 1), which indicates the absence of toxicity during this phase and also confirms the lack of estrogenic activity, since the presence of this activity in plant’s extracts can inhibit up to 100 % of the implantations in female rats, as was observed by Jagadish for Calotropis procera.30 It also confirms the lack of antiestrogenic activity, since the antiestrogenics substances administered to the rats during the first three days of pregnancy present anti-implantation effect.31 According to Gandhi, plants that exhibit antiestrogenic activity have the capacity of interrupting the gestation in rats and mice, by means of the inhibition of the estrogen necessary for the implantation in several species, including non-humans.32
As can be observed in table 2, besides the means of the implantation number, the number of live fetuses, mean weight of the fetuses and mean weight of the placentas, in the control and extract groups, did not present a statistically significant difference between the treatments, not showing morphological or functional alterations on the mothers of on the fetuses, demonstrating that there is no toxicity in the plant’s extract over gestation. That also demonstrates the lack of antiestrogenic activity, once the estrogens are necessary to the development of an environment appropriate for fertilization, implantation, nutrition of the embryo and parturition.33
In this study, the number of offspring in each litter was not changed by the treatment of the mothers with extract, suggesting that it does not exert toxicity during this phase.34 There was also no alteration in the weight of the newborn, showing a lack of toxicity during the growing phase. Congenital defects were not observed, what demonstrates the lack of teratogenic effects in the extract, indicating that the extract did not significantly change the immunological, endocrine, nutritional and the vascular aspects necessary to the growing and the normal development of the embryo and the fetuses.35
The pattern of events in the estrous cycle provides an useful indicator of the normality of the neurocrine function of the non pregnant females, its knowledge permits that the most favorable moment for mating be monitored, as well as the evaluation of the hormonal cycle based in the anatomical, histological and cytological alterations of the genital systems. The estrous cycle can be followed, in the rat, by observing the changes in the standard cytological vaginal smear.36,37 In this experiment, the frequency of appearance of each of the phases was evaluated and it was only observed a significant reduction in the number of estrus in the treatment and pos-treatment periods, indicating a toxic effect of the extract on follicular growing mechanism and/or ovulation (table 2).
The regular duration of the estrous cycle of the rat, represented by the interval between estrus, varies between 98 to 106 h, while the proestrus varies between 12 to 14, the estrus between 25 to 27, the metestrus between 6 to 8 and the diestrus 55 to 57 h.38 In table 2, a mean duration of the estrous cycle of 81,1 h was observed during the pretreatment period, 111.4 during the treatment and of 106,1 in the pos-treatment period, being observed a significant raise of the duration of the estrous cycle (p < 0.05) during the treatment and pos-treatment period, which can be related to the decrease of the follicular growth.24
The histopathological analysis did not reveal the signs of degenerative, inflammatory or necrotic alterations, be in the heart, liver or kidneys in the female pregnant rats from the groups treated with the extract in relation to the control group. This shows that the extract did not change the morphological structures of these organs, suggesting the absence of systemic toxicity, considering that they are very effused and exposed to substances present in the circulation.
In synthesis, the extract from the leaves of C. macrophyllum Tul, used orally in castrated female rats did not present estrogenic activity on the uterus, did not affect the pregnant rats in the litter’s uterine development, did not produce histopathological alterations on the heart, kidneys and liver of pregnant female rats. However, it produced alteration of the estrous cycle, decreasing the number of estrus and raising the duration of the cycle. Considering this, the extract from the leaves of C. macrophyllum Tul, must have its use better evaluated, if it is to be recommended for oral therapy in women of reproductive age.
ACKNOWLEDGEMENTS
Dr. Maurício Teles Freire from the Department of Biology of the Federal University of Piauí, for the identification of the botanical material, to the grant’s organs for the scholarships given to Mariana H. Chaves and Jamylla Mirck Guerra de Oliveira and the financial help (CNPq, CAPES/PROCAD).
REFERENCES
1. Ministério do Meio Ambiente, dos Recursos Hídricos e da Amazônia. Legal. Primeiro relatório nacional para a Convenção sobre Biodiversidade Biológica – Brasil. Ministério do Meio Ambiente, dos Recursos Hídricos e da Amazônia Legal, Brasília;1998.
2. Torres AR, Oliveira RAG, Diniz MFFM, Araújo EC. Estudo sobre o uso de plantas medicinais em crianças hospitalizadas da cidade de João Pessoa: riscos e benefícios. Rev Bras Farmacogn. 2005;(15):373-80.
3. Sousa CD, Silva HR, Vieira GM, Ayres MC, Costa CD, Araújo DS, et al. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim Nova. 2007;30(2):351-55.
4. Brandão MG, Consenza GP, Moreira RA, Monte RL. Medicinal plants and other botanical products from the Brazilian Official Pharmacopeia. Rev Bras Farmacog. 2006;(16):408-20.
5. Brandão MG, Consenza GP, Moreira RA, Monte RL. Medicinal plants and other botanical products from the Brazilian Official Pharmacopeia. Rev Bras Farmacog. 2008;(18):127-34.
6. Goldwyn S, Lazinsky A, Wei H. Promotion of health by soy isoflavones: efficacy, benefit and safety concerns. Drug Metabolism and Drug Interaction. 2000;(17):261–89.
7. Suthar AC, Banavalikar MM, Biyani MK. Pharmacological activities of genistein, an isoflavone from soy (Glycine max): part Ianti- cancer activity. Indian Journal of Experimental Biology. 2001;(39):511–19.
8. Suthar AC, Banavalikar MM, Biyani MK. Pharmacological activities of genistein, an isoflavone from soy (Glycine max): part IIanti- cholesterol activity, effects on osteoporosis and menopausal symptoms. Indian Journal of Experimental Biology. 2001;(39):520–25.
9. Kuiper GG, Carlsson B, Gradien K. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1998;(70):138.
10. Tham DM, Gardner CD, Haskell WL. Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological and mechanistic evidence. J Clin Endocrinol Metab. 1998;(83):2223-35.
11. Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;(17):353-81.
12. Hegnauer R, Barkmeijer RJG. Relevance of seed polyssacarides and flavonóides for the classification of the Leguminosae: a chemotaxonomic approach. Phytochemistry. 1993;(34):3.
13. Sales BHLN. Flavonóides de Lonchocarpus subguacescens (Benth) – Leguminosae e síntese de α- Hidroxichalconas. [dissertation]. Campinas (SP): Instituto de Química da UNICAMP; 1994.
14. Santos CA, Torres KR, Leonart R. Plantas medicinais: Herbarium, flora et scientia. 2nd ed. São Paulo: Editora Ícone; 1988.
15. Rocha H, Silva CC, Neto LB, Lopes JA, Citó AM, Chaves MH. Constituintes químicos das cascas do caule de Cenostigma macrophyllum: ocorrência de colesterol. Quim Nova. 2007;30(8):1877-881.
16. Kang DG, Yin MH, Oh H, Lee DH, Lee HS. Inhibiting activities of the secondary metabolites of Phlomis brunneogaleata against parasitic protozoa and plasmodia enoyl-ACP reductase, a crucial enzyme in fatty acid biosynthesis. Planta Medica. 2004;(70):718.
17. Saponara R, Bosisio E. Inhibition of CAMP-Phosphodiesterase by biflavones of Ginkgo biloba in rat adipose tissue. Journal of Natural Products. 1997;(61):1386.
18. Rodrigues MD, Martins-da-Silva RC, Secco RDS. Caesalpinieae (Leguminosae-Caesalpinioideae) from the Experimental field of the Embrapa Eastern Amazon, Moju, Pará State, Brazil. Hoehnea. 2012;39(3):489-516.
20. Nascimento JM, Conceição GM. Plantas medicinais e indicações terapêuticas da comunidade quilombola olho d’água do raposo, Caxias, Maranhão, Brasil. BioFar. 2011;6(2):138-51.
21. Hanrahan JR, Chebib M, Davucheron NL, Hall BJ, Johnston GAR. Semi synthetic Preparation of amentoflavone: a negative modator at GABA receptors Bioorganic. Medicinal Chemistry Letters. 2003;(13):2281.
22. Silva MI, Gondim AP, Nunes IF, Sousa FC. Utilização de fitoterápicos nas unidades básicas de atenção à saúde da família no município de Maracanaú (CE). Rev Bras farmacogn. 1997;(16):455-62.
23. Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J Biol. 2001;(62):609-14.
24. US EPA. Guidelines for reproductive toxicity risk assessment. EPA/630/R-96/009: Washington;1996.
25. Zenick H, Clegg ED. Assessment of male reproductive toxicity: A risk of assessment approach. In: Principles and methods of toxicology. In: New York: Raven Press; 1989. p. 275-309.
26. Khera KS. Maternal toxicity of drugs and metabolic disorders: a possible etiologic factor in the intrauterine death and congenital malformations: a critique on human data. Rev Toxicol. 1987;(17):345-75.
27. Paria BC, Lim K, Das SK, Reese J, Dey SK. Molecular signaling in uterine receptivity for implantation. Cell & Develop Biology. 2000;(11):67-76.
28. Beaudoin AR. Embryology and teratology. In: Baker HJ, Lindsey JR, Weisbroth SH. The laboratory rat (Research application). New York: Academic Press. 1980;2:75-94.
29. Allen WR. Maternal recognition and maintenance of pregnancy in the mare. Anim. Reprod. 2005;2(4):209-23.
30. Jagadish VK, Rana AC. Preliminary study on fertility activity of Calotropis procera roots in female rats. Fitoterapia. 2002;(73):111-15.
31. Dao B. Anti-Implantation Activity of Antiestrogens and Mifepristone. Contraception. 1996;(54): 253-8.
32. Ghandi M, Lal R, Sankaranarayanan A, Sharma PL. Post-coital antifertility activity of Ruta gravoleolens in female rats and hamsters. Journal of Ethnopharmacology. 1991;(34):49-50.
33. Lindzey J, Korach K. Estrogen Action on the Female Reproductive Tract. In: Knobil E, Neill JD. Encyclopedia of Reproduction. San Diego: Academic Press; 1999. p. 79-86.
34. Frohberg H. An introduction to research teratology. In: Neubert D, Merker HJ, Kwasigroch TE. Methods in prenatal toxicology. Stuttgart: Georg Thieme Publisher; 1977. p. 1-13.
35. Chahoud I, Ligensa A, Dietzel L, Faqi AS. Correlation between maternal toxicity and embryo/fetal effects. Reprod Toxicol. 1999;(13):375-81.
36. Long JA, Evans HM. The oestrus cycle in the rat and its associated phenomena. Mem Univ Calif. 1922;(6):1-111.
37. Cooper RL, Goldman JM, Vandenbergh JG. Monitoring of the estrous in the laboratory rodent by lavage vaginal. In: Heidell, JJ, Chapin RE. Methods in Toxicology: Female Reproductive Toxicology. San Diego: Academic Press; 1993. p. 45-56.
38. Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In Knobill and Neill (Ed.). The Physiology of reproduction, 2nd ed. New York: Raven Press; 1994. p. 12.
Recibido: 26 de enero de 2014.
Aprobado: 21 de marzo de 2015.
Amilton Paulo Raposo Costa. Rua Sem Luis Mendes R. Gonçalves, 4403, CEP 64055-350.Teresina Piauí, Brazil.
Correo electrónico: amilfox@uol.com.br