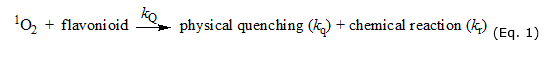Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Plantas Medicinales
versión On-line ISSN 1028-4796
Rev Cubana Plant Med vol.21 no.3 Ciudad de la Habana jul.-set. 2016
Rev Cubana Plant Med. 2016;21(3)
ARTÍCULO ORIGINAL
Kinetic study of the quenching of singlet oxygen by naringin isolated from peels of the fruit of bitter orange (Citrus aurantium I.)
Cinética del Quenching del oxígeno singulete por naringina aislada de cáscaras del fruto de naranja agria (citrus aurantium l.)
Carlos E. Diaz-Uribe,I Grace Oliveros,I Amner Muñoz-Acevedo,II William A. Vallejo LozadaI
I Grupo de Investigación en Fotoquímica y Fotobiología, Kilómetro 7 vía Puerto Colombia. Colombia.
II Grupo de Investigación en Química y Biología. Kilómetro 5 vía Puerto Colombia. Colombia.
ABSTRACT
Introduction: antioxidant activity is the capacity of a substance to inhibit oxidative degradation, mainly through its ability to react with both radical and non-radical species (e.g. singlet oxygen). Interest by scientific communities in the study of the antioxidant capacity of natural compounds has increased in recent years, due to their possible applications in the pharmaceutical, cosmetic and food industries.
Objective: estimate the antioxidant capacity of naringin against singlet oxygen using the rubrene method.
Methods: naringin was isolated from peels of the fruit of bitter orange (Citrus aurantium) and characterized using several spectroscopic techniques (UV-Vis and FTIR). The global rate constant for the reaction of 1O2 with naringin was determined with the Stern-Volmer plot derived from a stationary kinetic state based on the competition reaction with rubrene.
Results: results showed that naringin acted as singlet oxygen quenching agent with a global rate constant of 2.1 x 107 M-1s-1 (derived from the linear relationship of Stern-Volmer).
Conclusion: the kinetic study conducted suggests that naringin could be used as a singlet oxygen quenching agent in biological systems to protect them from oxidative damage.
Keywords: naringin, singlet oxygen, rubrene, Citrus aurantium.
RESUMEN
Introducción: la actividad antioxidante es la capacidad de una sustancia para inhibir la degradación oxidativa y actúa principalmente a través de su capacidad para reaccionar con las especies de radicales y no radicales (por ejemplo, oxígeno singulete). En los últimos años, se han incrementado el interés de las comunidades científicas en el estudio de la capacidad antioxidante de los compuestos naturales debido a sus posibles aplicaciones en la industria farmacéutica, cosmética y alimentaria.
Objetivo: estimar la capacidad antioxidante de la naringina contra el oxígeno singulete usando el método de rubreno.
Métodos: la naringina se aisló de cáscaras del fruto de la naranja agria (Citrus aurantium) y se caracterizó por algunas técnicas espectroscópicas (UV-Vis y FT-IR). La constante de velocidad global para la reacción de 1O2 con la naringina se determinó por medio del gráfico de Stern-Volmer derivado de una cinética en estado estacionario basada en la reacción de competición con el rubreno.
Resultados: los resultados mostraron que la naringina actuó como un quenching del oxígeno singulete con una constante de velocidad global de 2.1 x 10 7 M-1s-1 (derivado de la relación lineal de Stern-Volmer).
Conclusión: el estudio cinético sugiere que la naringina se podría utilizar como un quenching del oxígeno singulete en sistemas biológicos y protegerlos del daño oxidativo.
Palabras clave: naringina; oxígeno singulete; rubreno; Citrus aurantium.
INTRODUCTION
Research about singlet oxygen has been focused on specific chemical species in the medical sciences with regard to disease factors and health maintenance.1-3 Singlet oxygen reacts with many kinds of biological targets including lipids, proteins, DNA, and RNA4,5 inducing the deterioration of biological systems. This degradation is associated to different pathological events such as pigmentation, cataract, skin aging, and cancer.6-8 Currently, it is well known the different routes to produce singlet oxygen in biological systems, e.g., photosensitization9 and oxidative endogenous processes.10
Several natural antioxidants (e.g., carotenoids, vitamins and flavonoids), constituents of fruits and vegetables11, "quench" to singlet oxygen through the physical and chemical mechanisms. Flavonoids are natural phenolic compounds12 with antioxidant properties defined: the reaction mechanisms of these compounds on singlet oxygen have been reported (Eq. 1).13,14
Where kQ is the overall rate constant for the reaction of 1O2 with flavonoids (=kq + k r), kq and kr are the rate constants for the physical quenching and chemical reaction, respectively.
Naringin (4´,5,7-trihydroxyflavanone-7-b-L-rhamnoglucoside-(1,2)- a-D-glucopyranoside) is one flavanone found in citrus fruits. This compound has showed biological properties such as anti-inflammatory, anti-carcinogenicity5 and neuro-protective effect.6
In the scientific literature consulted, only one kinetic report (Montenegro et al.15) about the quenching of singlet oxygen (photo-chemically generated) by naringin was found. In this work, we studied antioxidant capacity of the naringin against singlet oxygen (chemically generated) using the rubrene method. Here, naringin was isolated from fruit peels of bitter orange (Citrus aurantium). The overall rate constant was determined from the steady-state treatment.
METHODS
Reagents and equipment
All reagents (ethanol, dichloromethane, butanol, sodium dodecyl sulphate, sodium molybdate, hydrogen peroxide, rubrene) used in this work were analytical grade. UV-Vis and FT-IR (KBr) spectra were obtained by Hewlett-Packard 8453 spectrophotometer, Bruker Tensor 27 spectrometers, respectively.
Isolation of naringin from Citrus aurantium
Citrus aurantium (bitter orange) fruits, in fresh and ripe state, were collected from Baranoa (latitude 10º 73' 33", longitude 74º 91' 66" - Atlántico, Colombia) between April and May 2013. After washing the fruits, the flavedo was mechanically separated of the albedo. Naringin was isolated from fruit peels according to the procedure described by Ikan16. After isolation, the solid was chemically characterized by UV-Vis and FT-IR techniques.
Generation and quenching of singlet oxygen
Singlet oxygen was generated by the chemical reaction of Na2MoO4 and H2O2, in the darkness, based on the methodology proposed by Aubry and Bouttemy17. The oil/water microemulsion (inverted micelle) was prepared from Na2MoO4.2H 2O (0.2 M, 6.0 mL), SDS (4.7 g), 1-butanol (9.4 g), and dichloromethane (60.0 mL), with magnetic stirring at room temperature (25 °C). Then, rubrene (0.2 mmol)/naringin (0.0 mM to 8.4 mM) and the microemulsion (15 mL) were introduced into a batch reactor with stirring. Subsequently, H 2O2 (15 %, 10 μL) were added to each solution. The solutions with rubrene were monitored at 522 nm.
Determination of overall singlet oxygen quenching rate
The overall rate constant kQ (=kq + kr) for the reaction of 1O2 with naringin was determined by means of a Stern-Volmer plot derived from steady state kinetic based on the competition reaction using rubrene (standard compound) and ethanol (solvent) at 25 °C. The estimated experimental error for the rate constant (kQ) was less than five percent (5 %).
RESULTS
Characterization of isolated naringin
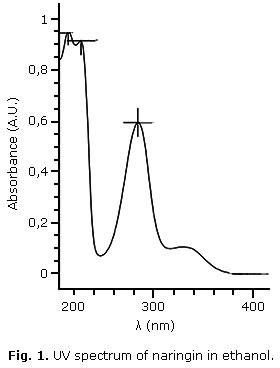
The UV spectrum of naringin in ethanol is shown in Fig 1. Naringin exhibits two major absorption bands in the UV region, at 284 nm (band I) representing B-ring absorption (cinnamoyl system), and at 229 nm (band II), considered to be associated with the absorption involving the A ring benzoyl system, this is a typical result for flavones and flavonols.18
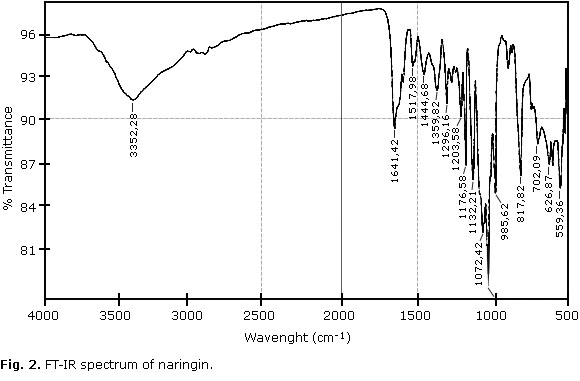
The FT-IR spectrum of naringin is shown in Fig 2. This spectrum is characterized by vibrational bands mainly due to the OH, C-H, C=O, and C=C functional groups. The u (OH), u (C-H), u (C=O), u (C=C) for naringin appear at 3425 cm-1, 2957-2859 cm-1, 1645 cm-1, respectively.18
Overall rate constants (kQ) for the reaction of 1O2 with naringin
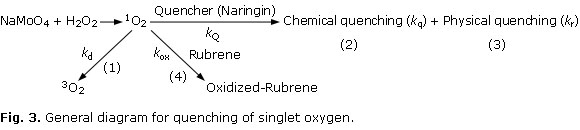
Singlet oxygen could disappear by 4 routes (reactions 1 to 4, Fig 3): non-radiative decay (1), singlet oxygen chemical quenching (2), singlet oxygen physical quenching (3) and reaction with rubrene (4).
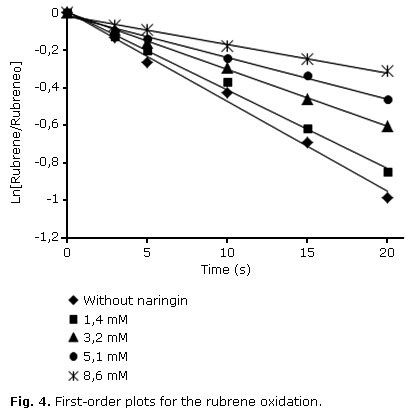
In this study, two solutions of equal volume, the first containing naringin and the latter without a quencher and each one having the same initial concentration of rubrene, were exposed to the same amount of singlet oxygen. Fig 4 shows the effect of naringin on rubrene oxidation induced by singlet oxygen in ethanol.
The results showed that naringin inhibited the oxidation of rubrene efficiently in a concentration-dependent manner; besides, the linear fitting of the plots indicated that rubrene oxidation followed a first-order kinetics.
The overall rate constant kQ (=kq + kr) for the reaction of 1O2 with naringin was determined in ethanol by Eq. 2 derived from the steady-state treatment of Fig 3.
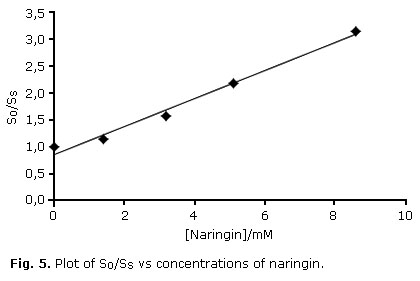
S 0 /SS = 1 + [(kq + kr)/kd][Naringin] (Eq. 2)
Where S0 and SS are slopes of the first-order plots of disappearance of 1O2 acceptor, rubrene, in absence and presence of naringin, respectively. kd is the rate of deactivation of 1O2 in ethanol. A plot of S0/SS vs concentration of naringin is shown in Fig 5. The overall rate constant (kQ) was calculated by using the value of kd in ethanol (kd= 8.3 x 104 s-1), reported by Merkel and Kearns.19
The kQ value obtained was 2.1 x 107 M-1s-1. This result indicated that the naringin could act as scavenger of 1O2. The quenching process probably occurs via reversible charge transfer with the formation of an "exciplex" (excited complex): the mechanism involves an excited state encounter complex with a partial character of charge transfer, in which the 1O2 is the acceptor species.20,21
DISCUSSION
The flavonoids act as quenchers of singlet oxygen; Tournaire et al.13 reported that the efficiency of the physical quenching (k q) is mainly controlled by the presence of a catechol moiety on ring B, whereas the structure of ring C (particularly the presence of a hydroxyl group activating the 2,3-double bond) is the main factor determining the efficiency of the chemical reactivity (kr) of flavonoids with 1O2. However, the chemical reaction is almost negligible due to kQ»kq. The total reactivity scale (kQ) is dominated by kq, which is in general higher than kr.
Nagai et al.21 determined the rate constants of flavonoids without a 2,3-double bond and studied the effect of p-conjugation between A- (resorcinol) and B- (phenol) rings on the reaction rates. In this work was found that the 4'-OH group in the B-ring contributed to the 1O 2 quenching action by flavonoid, while the resorcinol structure in the A-ring contributed partly (4-17%) to the 1O2 quenching. The overall rate constant (3.7 x 106 M-1s-1) for the reaction of 1O2 with naringenin reported was smaller than the value obtained (2.1 x 107 M-1s-1) for naringin in this work; this could be due to that naringin is the glycosilated naringenin (flavanone) and the sugar molecules could be contributing to the 1O2 quenching, through stabilization of excited complex formed. Nonetheless, the quenching rates of a-tocopherol (2.1 x 108 M-1s-1) and b-carotene (1.6 x 1010 M-1s-1), the main quenchers of 1O2 22,23, are one to three orders of magnitude higher than that of naringin. On the other hand, the rate constants (kQ) for some flavonoids are resemble to naringin: chrysin (2.0 x 107 M-1s-1), apigenin (2.8 x 107 M-1s-1), rutin (1.2 x 108 M -1s-1), quercetin (4.6 x 108 M-1s-1), myricetin (5.1 x 108 M-1s-1), catechins (1.1 x 107 M-1s-1 - 1.5 x 108 M-1s-1) 21. Finally, it is worth mentioning that the synthetic antioxidants BHA (terc-butylhydroxyanisol, 3.4 x 107 M-1s-1) and BHT (terc -di-butylhydroxytoluene, 4.3 x 106 M-1s-1) 24 have singlet oxygen quenching activities comparable to naringin.
Most reactions of 1O2 with biological targets (lipids, proteins (amino acids), DNA, and RNA) occur per chemical via rather than physical route inducing to cell death and mutations 4,5. For instance, the quenching rates for stearic, oleic, and linoleic acids, cholesterol and DNA 21 are 9.0 x 103 M-1s-1, 1.7 x 104 M-1s-1, 4.2 x 10 4 M-1s-1, 5.7 x 104 M-1s-1, and 5.1 x 105 M-1s-1, in that order. The kQ value (2.1 x 107 M-1s-1) determined for naringin was two to four orders of magnitude higher than those fatty acids and DNA. This result suggests that naringin could contribute to the protection of the lipid peroxidation and the degradation of DNA in photosynthetic systems, by quenching 1O2. Finally, our results are relevant and important due to that the naringin was obtained from a low-cost source such as orange peel wastes and, this implementation could be used to diminish the environmental impact by accumulation of these natural residues.
In this work, we isolated naringin from C. aurantium, the analysis through UV and FT-IR confirmed the identity of compound. Furthermore, we showed that naringin is a quencher of singlet oxygen, one of the most important reactive oxygen species. The naringin inhibited the rubrene oxidation by singlet oxygen and the overall rate constant in ethanol was 2.1 x 107 M-1s-1. The kinetic study suggests that the naringin could be used as a singlet oxygen quencher in biological systems and protect them of oxidative damage. Finally, we found a practical application for orange peel waste, an low-cost and abundant resource.
Acknowledgments - A-MA thanks to Universidad del Norte for the financial support through of the Strategic Area "Biodiversidad, Servicios Ecosistémicos y Bienestar Humano" (Code Project 2013-DI0024).
REFERENCES
1. Schrag M, Mueller C, Zabel M, Crofton A, Kirsch WM, Ghribi O, et al. Oxidative stress in blood in Alzheimer's disease and mild cognitive impairment: A meta-analysis. Neurobiol. Dis. 2013;59(0):100-10.
2. Stief TW. The physiology and pharmacology of singlet oxygen. Med. Hypotheses. 2003;60(4):567-72.
3. Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell B. 2012;42(10):1634-50.
4. Ray RS, Mujtaba SF, Dwivedi A, Yadav N, Verma A, Kushwaha HN, et al. Singlet oxygen mediated DNA damage induced phototoxicity by ketoprofen resulting in mitochondrial depolarization and lysosomal destabilization. Toxicology. 2013;314(0):229-37.
5. Wenli Y, Yaping Z. Chemiluminescence evaluation of oxidative damage to biomolecules induced by singlet oxygen and the protective effects of antioxidants. Biochim. Biophys. Acta. 2005;1725(1):30-4.
6. Wen X, Wu J, Wang F, Liu B, Huang C, Wei Y. Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free Rad. Biol. Med. 2013;65(0):402-10.
7. Mehdi MM, Rizvi SI. Plasma protein hydroperoxides during aging in humans: correlation with paraoxonase 1 (PON1) arylesterase activity and plasma total thiols. Arch. Med. Res.2013;44(2):136-41.
8. Zigler J, Goosey JD. Singlet oxygen as a possible factor in human senile nuclear cataract development. Curr Eye Res. 1984;3(1):59-65.
9. Kanofsky J.R. Singlet oxygen production by biological systems. Chem Biol Interact. 1989;70(1-2):1-28.
10. Glette J, Sandberg S. Phototoxicity of tetracyclines as related to singlet oxygen production and uptake by polymorphonuclear leukocytes. Biochem Pharmacol. 1986,35(17):2883-85.
11. Harasym J, Oledzki R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition. 2014;30(5):511-17.
12. Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Science. 2012;196(0):67-76.
13. Tournaire C, Croux S, Maurette MT, Beck I, Hocquaux M, Braun AM, et al. Antioxidant activity of flavonoids: Efficiency of singlet oxygen (1 Δg) quenching. J. Photochem. Photobiol. B: Biol.1993;19(3):205-15.
14. Mukai K, Nagai S, Ohara K. Kinetic study of the quenching reaction of singlet oxygen by tea catechins in ethanol solution. Free Rad. Biol. Med. 2005; 39(6):752-61.
15 Montenegro MA, Nazareno MA, Borsarelli CD. Kinetic study of the photosensitized oxygenation of the flavanone naringin and its chalcone. J. Photochem. Photobiol. A: Chem. 2007;186(1):47-56.
16. Ikan R. Acetogenins in Natural Products. London and New York: Academic Press; 1969.
17. Aubry JM, Bouttemy S. Preparative oxidation of organic compounds in microemulsions with singlet oxygen generated chemically by the sodium molybdate/hydrogen peroxide system. J. Am. Chem. Soc. 1997;119(23):5286-94.
18. Özyürek M, Akpınar D, Bener M, Türkkan B, Güçlü K, Apak R. Novel oxime based flavanone, naringin-oxime: Synthesis, characterization and screening for antioxidant activity. Chem-Biol. Interact. 2014;212(0):40-46.
19. Merkel PB, Kearns DR. Radiationless decay of singlet molecular oxygen in solution. Experimental and theoretical study of electronic-to-vibrational energy transfer. J. Am. Chem. Soc.1972;94(21):7244-53.
20. Thomas MJ, Foote CS. Chemistry of singlet oxygen-XXVI. Photooxygenation of phenols. Photochem. Photobiol. 1978;27(6):683-93.
21. Nagai S, Ohara K, Mukai K. Kinetic Study of the Quenching Reaction of Singlet Oxygen by Flavonoids in Ethanol Solution. J. Phys. Chem. B. 2005; 109(9):4234-40.
22. Mukai K, Ouchi A, Nakano M. Kinetic study of the quenching reaction of singlet oxygen by pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in micellar Solution. J. Agric. Food Chem. 2011,59(5):1705-12.
23. Ouchi A, Aizawa K, Iwasaki Y, Inakuma T, Terao J, Nagaoka SI, et al. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. Developmentof a Singlet Oxygen Absorption Capacity (SOAC) Assay Method. J. Agric. Food Chem. 2010;58(18):9967-78.
24. Kim JI, Lee JH, Choi DS, Won BM, Jung MY, Park J. Kinetic study of the quenching reaction of singlet oxygen by common synthetic antioxidants (tert-butylhydroxyanisol, tert-di-butylhydroxytoluene, and tert-butylhydroquinone) as compared with α-Tocopherol. J. Food Sci. 2009,74(5):362-69.
Recibido: 31 de julio de 2015.
Aprobado: 1 de abril de 2016.