My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Ciencias Médicas de Pinar del Río
On-line version ISSN 1561-3194
Rev Ciencias Médicas vol.26 no.4 Pinar del Río July.-Aug. 2022 Epub July 01, 2022
Articles
Nimotuzumab in patients with COVID-19 in Pinar del Río
1 University of Medical Sciences of Pinar del Río. Clinical Surgical Teaching Hospital "Dr. León Cuervo Rubio". Pinar del Río, Cuba..
Introduction:
COVID-19 is an emerging viral disease of the XXI century, being a respiratory disease that can present in severe forms due to the state of systemic hyperinflammation generated by it, for which several treatments have been used in Cuba to improve respiratory ventilation, avoid hyperinflammation and prevent sequelae such as pulmonary fibrosis, where one of these drugs has been the monoclonal antibody Nimotuzumab; for playing an important role against the epidermal growth factor receptor.
Objective:
to characterize the use of Nimotuzumab in patients with COVID-19 in the Clinical Surgical Teaching Hospital "León Cuervo Rubio" of Pinar del Río, from July to October 2021.
Methods:
an observational, descriptive and transversal study was carried out in patients with COVID-19 treated at the "León Cuervo Rubio" Clinical Surgical Teaching Hospital. A total of 419 patients were studied, selected by simple random sampling, where their clinical histories were reviewed.
Results:
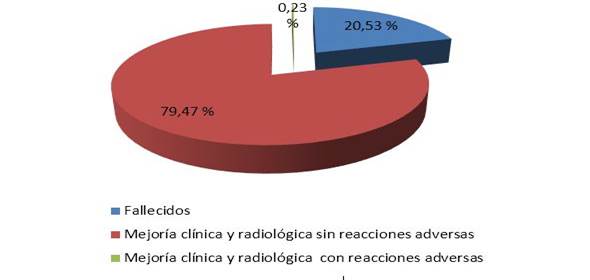
53,59 % were male, between 60 and 79 years old (45,82 %). The risk factors with the highest incidence were arterial hypertension (71,36 %) and diabetes mellitus (21,96 %). One patient was reported with adverse reactions with chills and fever and 79,47 % recovered, with 20,53 % dying.
Conclusions:
the predominant sex was male, between 60 and 79 years old, with risk factors of arterial hypertension and diabetes mellitus; where both clinical and radiological recovery was excellent and with high drug safety.
MeSH: PNEUMONIA; COVID-19; PROTOCOLS
INTRODUCTION
The current global COVID-19 infection caused by the new coronavirus (SARS-CoV-2), since the first cases reported in Wuhan city on August 31, 2019, in China, infected millions of people worldwide. This new pandemic is characterized by acute respiratory distress syndrome (ARDS), multi-organ failure, and other severe complications.1,2
It is an enveloped RNA virus,3 which is transmitted by respiratory droplets; this is the main mode of transmission by direct contact and also by indirect contact with an infected person or by contaminated hands passed through the mucous membranes of the oral cavity, nose and eyes. The incubation period is from one to 14 days and the transmissibility period up to 14 days after the disappearance of symptoms; while the clinical picture of the cases presents with fever and some patients present dyspnea and pneumonic changes on chest radiographs with images that may be localized or affect one or more lung fields, unilateral and bilateral.4,5,6
The main clinical forms recognized by the World Health Organization are uncomplicated (minimally symptomatic) disease, uncomplicated uncomplicated lower respiratory tract infection (mild pneumonia), severe pneumonia with or without acute respiratory distress syndrome (ARDS), sepsis, or septic shock.7
The virus affects more severely people at advanced ages, patients with immunosuppression and chronic diseases such as diabetes mellitus, ischemic heart disease, cancer, chronic lung disease and obesity. Among the most frequent complications are: acute respiratory distress syndrome and cytochemical storm syndrome; they can usually occur from the seventh day of onset of symptoms.8
The calculated lethality is approximately 2 to 4 % worldwide, the countries with the highest tansmissibility and mortality are the countries of America, especially the United States and Brazil, which account for more than 30 % of those infested worldwide.7
In the statistical yearbook of the Ministry of Public Health of Cuba,9 it was reported that between 2020, 1.3 per 100,000 inhabitants in Cuba had been infested with this disease with 143 deaths, which has been higher in the year 2021 for having presented the maximum peak of this epidemiological situation.
With the appearance in Cuba of the first cases of the disease, on March 11, 2020, the Cuban Minister of Public Health established a "sanitary emergency" and coincided with the declaration of a pandemic worldwide.10 From that moment on, as part of the plan to confront this nosological entity, a management group was created, composed of leaders and scientists, to confront this emerging disease. National action protocols were created with drugs produced in the country, based on the knowledge acquired, which have undergone modifications throughout this pandemic.
Several drugs of national production have been used for this disease, one of them is nimotuzumab, which was introduced in version 1.6 for moderate risk (phenotype IV) and severe (phenotype V) patients in the Cuban National Protocol since last June 2021.8
Nimotuzumab (TheraCIM®) is a humanized antibody against the epidermal growth factor receptor (EGF-R), generated by transplantation of the complementarity determining regions or hypervariable regions of the murinoioregf/r3 antibody, in a human immunoglobulin support framework. The humanized antibody recognizes the receptor with similar affinity to its (10-9M) ligands, is able to bind to the extracellular domain of EGF-R with high affinity and, in turn, strongly inhibits the signaling pathway associated with this receptor.
Given that this virus and its infection in the lung induce this same receptor and its signaling cascade aggravates the inflammatory process which could impact on the pathogenesis of the disease; in May 2021 a study was carried out in 40 patients in Havana City, it was concluded that it improved respiratory ventilation, prevented hyperinflammation and sequelae such as pulmonary fibrosis.11,12
This nosological entity was during the years 2020 -2021 the first cause of admission and death in the Clinical Surgical Teaching Hospital "León Cuervo Rubio" of Pinar del Río.13 As in the rest of the country, the incidence of this disease has increased in the province in the last six months. However, with the introduction of nimotuzumab to the national protocol and with its distribution, it is intended to expose the experiences obtained in the Pinar del Río population in patients with severe presentations of COVID-19.
METHODS
A single-center, observational, descriptive, descriptive, cross-sectional study was carried out in patients attended at the "León Cuervo Rubio" Clinical Surgical Teaching Hospital of Pinar del Río, between July and October 2021, in serious condition with a diagnosis of COVID-19 confirmed by polymerase chain reaction (PCR). The universe consisted of patients with a diagnosis of COVID-19 with treatment with nimotuzumab, for a sample of 419, selected by simple random sampling.
To obtain the information, the individual clinical histories of each patient classified as moderate risk (phenotype IV) and severe (phenotype V) were reviewed; once three doses of the monoclonal antibody, nimotuzumab (50 mg Bbos), were applied every 72 hours, with a first dose of 200mg of the product and the other two doses with 100mg in infusion to last two hours. Variables such as age, sex, risk factors, adverse reactions and clinical-radiological results after the drug were analyzed.
Descriptive statistics were used for data analysis, by calculating absolute and relative percentage frequencies.
The principles of medical ethics and the aspects established in the Declaration of Helsinki were complied with.
RESULTS
Male sex predominated with 53,59 %, ages between 60 and 79 years were the most frequent for both sexes with 45,82 %.
Table 1 Distribution of patients with COVID - 19 treated with nimotuzumab according to age groups and sex, admitted to the Hospital Clínico Quirúrgico Docente Dr. León Cuervo Rubio, Pinar del Río, between July and October 2021.
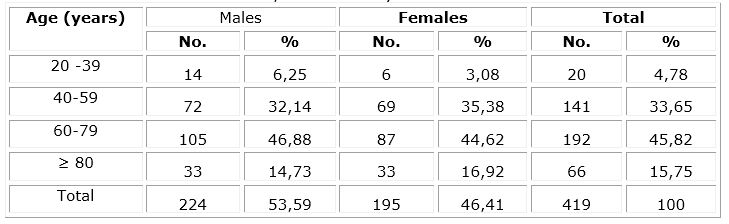
Source: Medical records.
Predominance among the risk factors was found to be arterial hypertension (71,36 %) and diabetes mellitus (21,96 %).
Table 2 Distribution of patients with COVID - 19 treated with nimotuzumab according to risk factors.
| Risk Factors | No. | % |
|---|---|---|
| Obesity | 67 | 15,99 |
| Arterial hypertension | 299 | 71,36 |
| Diabetes Mellitus | 92 | 21,96 |
| COPD | 11 | 2,63 |
| Ischemic heart disease | 31 | 7,40 |
| Cerebrovascular disease | 10 | 2,39 |
| Bronchial Asthma | 20 | 4,77 |
| Parkinson's disease | 2 | 0,48 |
| Chronic renal insufficiency | 3 | 0,72 |
| Cancer | 6 | 1,43 |
| Hypothyroidism 4 0.95 | 4 | 0,95 |
| Gout | 6 | 1,43 |
| Rheumatoid arthritis | 5 | 1,19 |
Source: Medical records.
There was a predominance of patients who had clinical and radiological improvement once the drug was administered (79,.47 %). One patient was reported with adverse reactions (chills and fever).
DISCUSSION
In recent years, COVID-19 has become the battle for life for all the nations of the world; therefore, governments have tried to fight against the SARS-CoV-2 virus, responsible for the second pandemic of the 21st century.10 Cuba has made every effort to fight against the SARS-CoV-2 virus.
Cuba has made every effort to combat this situation and has dedicated to this work dissimilar resources, both material and human, hence the creation of national action protocols that are subject to constant changes thanks to the study of this virus by dissimilar scientists.6
This study coincides with studies carried out by Dr. Moreno-Gonzalez et al.6 where 56 % of the patients who died worldwide were men.
Men are vulnerable to this disease because they spend more time on the streets. Women, on the other hand, are to some degree more protected because, as part of the protection provided to minors, the Cuban government established a law that allows mothers to stay at home with their children.
Although the disease does not behave in the same way as in its beginnings, (the incidence was higher in elderly patients) since there is no difference in the ages that present the severe forms, even those over 60 years of age are more vulnerable. Therefore, this study coincides with what was stated by Peña Otero,1 when observing behavior of this new virus at world level (Table 1).
The researchers of this article consider that, due to the consequences of aging on the immune system, the propensity to suffer non-communicable chronic diseases that predispose to tissue pre-inflammation; those over 60 years of age are more easily complicated.
This infectious agent affects patients who are previously inflamed, due to other diseases prior to its arrival in the organism, such as arterial hypertension, diabetes mellitus, obesity, chronic renal insufficiency, chronic obstructive pulmonary disease, cancer, among others.8
The unfavorable effects of these diseases, especially diabetes and obesity, on the course of viral infections have been attributed to metabolic degradation and chronic inflammation of adipose tissue deposits, leading to activation of blunted macrophages and impairment of T and B lymphocyte responses.10
Such morbidities systematically induce chronic inflammation by increasing the secretion of cytokines, such as interleukin 6 (IL6), interleukin 8 (IL8) and tumor necrosis factor α, which may aggravate damage to the lung parenchyma and bronchi. This inflammation may worsen the acute inflammatory response triggered by a SARS-CoV-2 infection, which may be associated with a cytokine release syndrome.10
This explains what was evidenced in the research, which also coincides with the study conducted in publications of several international journals, by Dr. Franklin Aguilar-Gamboa et al.10 where he sees obesity and diabetes as predictors of complication before the disease; 28,2 % of the severe cases had these criteria.
With the actions generated by these nosological entities in the organism prior to infection by COVID-19, this virus, within its pathogenesis in the second week of infection, produces the same effect as chronic diseases, so that in these patients the situation is aggravated.
Treatments for COVID-19 are focused on reducing symptoms, the use of antivirals, anti-inflammatory drugs, monoclonal antibodies and COVID-19 convalescent plasma has shown variable results.5
Monoclonal antibodies, such as Tocilizumab and Sarilumab (trade names), which are used to inhibit the synthesis of inflammatory cytokines, thereby decreasing the "cytokine storm", which is a critical condition reported in severe COVID-19 patients and cause of subsequent mortality, have been used worldwide as a therapeutic alternative to avoid the complications of this disease.5
Knowing the pathogenic element of the disease, Cuban scientists looked for alternative Cuban drugs that would act at this level to avoid the evolution of the disease to severe processes or to stop their evolution, with the use of an anti-EGFR monoclonal antibody, of intermediate affinity designed by genetic engineering, which inhibits cell proliferation and active natural killer cell angiogenesis, such as Nimotuzumab (CIMAher®), which was discovered and approved in 2002 for head and neck cancer, as well as esophageal, lung, pancreatic, hepatocarcinoma, colonorectal oncogenic diseases and brain metastases; and has a good safety and effectiveness profile. 14,15,16,17,18
It was used for the first time in Havana at the Salvador Allende Hospital in 40 patients with COVID-19 with encouraging results, where the patients who were included in this study mostly had chronic cormobilities like those in this study and it was evidenced according to Dr. Tania Crombet Ramos. Tania Crombet Ramos, doctor of the Molecular Engineering Center and member of the study of this drug, that this antibody improved ventilatory function in these patients, decreased pulmonary fibrosis and stabilized interstitial pneumonia on the seventh day, which coincides with this work.
The human body naturally produces antibodies to fight infections; but this virus, like all others, circumvents the natural defenses and makes the antibodies sick.15
The artificial antibody can produce adverse reactions as an external agent that enters the human organism and modifies cellular structures; which can range from chills, fever, general malaise, digestive symptoms, alopecia, headache, and others.9
In studies carried out by Amaró Garrido et al.5 in patients with lung cancer, only 152 patients had adverse reactions for 3,9 % and it is thought that the underlying disease had a great influence because they had brain metastasis; which coincided with this study.
The researchers consider that the improvement with the application of this product is due to the similarity in the pathogenesis of this disease and the blocking effect generated by this drug. While it is a harmless product because it does not generate hypermagnasemia like the rest of the monoclonal antibodies existing in the world. If the symptomatology of the high concentration of magnesium in the blood is reviewed, it generates the side effects described by these products.
The predominant sex was male, between 60 and 79 years of age, with risk factors of arterial hypertension and diabetes mellitus, with high safety to the drug, while the results obtained in terms of clinical and radiological improvement were impressive; pulmonary fibrosis and mortality in severe COVID-19 patients decreased.
REFERENCES
1. Peña Otero D. Manejo clínico del COVID-19: unidades de cuidados intensivos. Ministerio de Sanidad del Gobierno de España. [Internet]. España; 2020 [citado 23/05/2020]: [14 pp.]. Disponible en: Disponible en: https://www.researchgate.net/publication/340416345_ [ Links ]
2. Ministerio de Salud Pública. Protocolo de actuación nacional para la covid-19. Versión 1.6. [Internet]. 2021 [citado 23/08/2021]: [aprox. 64p]. Disponible en: Disponible en: https://salud.msp.gob.cu/protocolo-de-manejo-clinico-de-covid-19-version-1-6/ [ Links ]
3. Quiñones-Laveriano DM, Soto A, Quilca-Barrera L. Frecuencia de coinfección por patógenos respiratorios y su impacto en el pronóstico de pacientes con COVID-19. Rev. Fac. Med. Hum [Internet]. 2021 jul/set [citado 09/09/2021]; 21(3): 610-622. Disponible en: Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S2308-05312021000300610&lang=es [ Links ]
4. de Carvalho Neto JN, Moreira Loiola B, Silva Rodrigues VE, Milanês Sousa LR, Negreiros AL. Resultados y características clínicas de personas con obesidad y covid-19: revisión integrativa. Enferm. glob. [Internet]. 2021 agosto 02 [citado 09/09/2021]; 20(63): 544-580. Disponible en: Disponible en: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1695-61412021000300017&lang=es [ Links ]
5. Amaró Garrido MA, Solenzal Álvarez YT, Hernández González T, Geovanis Alcides Orellana Meneses GA. Diagnóstico imagenológicos de neumonía por SARS-CoV-2 en pacientes con la Covid-19. Gac Méd Espirit [Internet]. 2020 diciembre 03 [citado 09/09/2021]; 22(3): 175-193. Disponible en: Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1608-89212020000300175&lang=es [ Links ]
6. Moreno-González JG, Siqueiros-Cendón T, Moreno-Brito V, Licón Trillo L, González-Rodríguez, Leal-Berumen I, et al. COVID-19, diabetes y el sistema inmunológico. Nova scientia [Internet]. 2021 mayo 28 [citado 09/09/2021]; 13(spe). Disponible en: Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-07052021000300102&lang=es [ Links ]
7. OPS. Inmunobiológicos (Anticuerpos) [Internet]. OPS; 2010 [citado 09/09/2021]: [aprox. 1 p.]. Disponible en: Disponible en: https://www3.paho.org/cub/index.php?option=com_content&view=article&id=239:inmunobiologicos-anticuerpos&Itemid=0 [ Links ]
8. Moreno S, Yepes D, Arias JH. Síndrome de dificultad respiratoria aguda en el contexto de la pandemia por COVID-19. CES Med. [internet]. 2020 [citado 09/09/2021]; 34(spe): 69-77. Disponible en: Disponible en: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-87052020000400069&lang=es [ Links ]
9. Ministerio de Salud Pública. Anuario estadístico de salud [Internet]. La Habana; 2021. [citada 09/09/2021]: 192p. Disponible en: Disponible en: https://salud.msp.gob.cu/wp-content/Anuario/Anuario-2020.pdf [ Links ]
10. Aguilar-Gamboa FR, Vega-Fernández JA, Suclupe-Campos DO. SARS-CoV-2: mucho más que un virus respiratorio. AMC [Internet]. 2021 mar/abr [citado 09/09/2021]; 25(2): e8018. Disponible en: Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1025-02552021000200014 [ Links ]
11. Aguilera Calvo N, del Cristo Domínguez IS, Muñoz Morejón Y, Palomino Machado L, Macías Abraham A. Evaluación de la seguridad del nimotuzumab en pacientes con cáncer de pulmón de células no pequeñas, portadores de metástasis cerebral. Gac Méd Espirit. [Internet]. 2018 set-dic [citado 09/09/2021]; 20(3). Disponible en: Disponible en: http://revzoilomarinello.sld.cu/index.php/zmv/article/view/311 [ Links ]
12. Chávez Fernández L, Noda Alonso SH. Realizan en Cuba estudio observacional del Nimotuzumab para lograr su uso de emergencia. [Internet]. Granma; 2021 Noviembre 11 [citado 21/11/2021]. En sección científica. Disponible en: Disponible en: http://www.cuba.cu/salu/2021-09-08/realizan-en-cuba-estudio-observacional-del-nimotuzimab-para-lograrsu-uso-de-emergencia-/57275 . [ Links ]
13. Concepción Y, Cruz Quesada JE. Boletín estadístico de los resultados de los indicadores hospitalarios del Hospital Clínico Quirúrgico Docente "León Cuervo Rubio" del año 2020. Acta 1 del Consejo de dirección del Hospital Clínico Quirúrgico Docente "León Cuervo Rubio". Pinar del Río; 2021 enero 30.p.12-19. [ Links ]
14. Leslie Pérez-Ruiz L, Rodríguez-Mendoza MM, Soto-Molina H, Galán-Álvarez Y, Viada-González CE, Collazo-Herrera MM. Nimotuzumab (CIMAher(r)) en pacientes cubanos con cáncer de cabeza y cuello estadios III/IV: Análisis de impacto presupuestario. Vaccimonitor [Internet]. 2020 ene - abr [citado 09/09/2021]; 29(1): 14-21. Disponible en: Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1025-028X2020000100014&lang=es [ Links ]
15. Gonzales Zamora JA, Quiroz T, Vega AD. Tratamiento exitoso con Remdesivir y corticoides en un paciente con neumonía asociada a COVID-19: reporte de un caso. Medwave [Internet]. 2020 [citada 09/09/2021]; 20(7): e7998. Disponible en: Disponible en: https://www.medwave.cl/medios/medwave/Agosto2020/PDF/medwave-2020-07-7998.pdf [ Links ]
16. Crombet-Ramos T, Rak J, Perez R, Viloria-Petit A. Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer [Internet]. 2002 [citado 09/09/2021]; 101(6): 567-75. Disponible en: Disponible en: https://onlinelibrary.wiley.com/doi/abs/10.1002/ijc.10647 [ Links ]
17. Cuba. RPCEC /CENCEC-MINSAP. Racotumomab, Nimotuzumab o Docetaxel para el tratamiento de cáncer de pulmón de células no pequeñas avanzado [Internet]. La Habana: Registro Público Cubano de Ensayos Clínicos; 2014 [citado 09/09/2021]. Disponible en: Disponible en: https://rpcec.sld.cu/ensayos/RPCEC00000179-Sp [ Links ]
18. Suarez Martínez G, Salva Camaño SN, Piedra Sierra P, Iglesias Castillo B, Toledo Jiménez C, Solomón Cardona MT, et al. Seguridad y efectividad del nimotuzumab en los pacientes con tumores gliales malignos. Rev Cubana Neurol Neurocir [Internet]. 2015 [citado 09/09/2021]; 5(2): 123-32. Disponible en: Disponible en: http://www.revneuro.sld.cu/index.php/neu/article/view/164/pdf [ Links ]
Received: December 06, 2021; Accepted: April 22, 2022











 text in
text in